Voltage-gated sodium channel Nav1.6 is modulated by p38 mitogen-activated protein kinase - PubMed (original) (raw)
Voltage-gated sodium channel Nav1.6 is modulated by p38 mitogen-activated protein kinase
Ellen K Wittmack et al. J Neurosci. 2005.
Abstract
Nav1.6 is the major sodium channel isoform at nodes of Ranvier in myelinated axons and, additionally, is distributed along unmyelinated C-fibers of sensory neurons. Thus, modulation of the sodium current produced by Nav1.6 might significantly impact axonal conduction. Mitogen-activated protein kinases (MAPKs) are expressed in neurons and are activated after injury, for example, after sciatic nerve transection and hypoxia. Although the role of MAPK in signal transduction and in injury-induced regulation of gene expression is well established, the ability of these kinases to phosphorylate and modulate voltage-gated sodium channels has not been reported. Sequence analysis shows that Nav1.6 contains a putative MAP kinase-recognition module in the cytoplasmic loop (L1), which joins domains 1 and 2. We show in this study that sodium channels and p38 MAP kinase colocalize in rat brain tissue and that activated p38alpha phosphorylates L1 of Nav1.6, specifically at serine 553 (S553), in vitro. None of the other cytoplasmic loops and termini of the channel are phosphorylated by activated p38alpha in these assays. Activation of p38 in the neuronal ND7/23 cell line transfected with Nav1.6 leads to a significant reduction in the peak Nav1.6 current amplitude, without a detectable effect on gating properties. The substitution of S553 with alanine within L1 of the Nav1.6 channel prevents p38-mediated reduction of Nav1.6 current density. This is the first demonstration of MAPK phosphorylation and modulation of a voltage-gated sodium channel, and this modulation may represent an additional role for MAPK in regulating the neuronal response to injury.
Figures
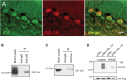
Figure 1.
MAP kinase p38 associates with voltage-gated sodium channels in rat brain. A, p38 (green) and Nav1.6 (red) immunofluorescent signals are present within cerebellar Purkinje cells; merged image (merge) demonstrates colocalization of p38 and Nav1.6 within Purkinje cells. Scale bar, 25 μm. B, MAP kinase p38 antibody was used to immunoprecipitate the voltage-gated sodium channels from a rat brain lysate. Anti-mouse IgG was used as a negative control to rule out nonspecific binding. Immunoblot analysis of the IP complex was performed using pan sodium channel antibody. Lane 1 shows a robust immunoreactive signal from the cell lysate that was used for the IP assay, consistent with the presence of intact sodium channel proteins in this sample. Nonspecific antibodies do not immunoprecipitate a channel complex (lane 2). Anti-P38 coimmunoprecipitated voltage-gated sodium channels from the brain lysate (lane3). Molecular weight marker in kilodaltons is shown on the left. C, Pan sodium channel antibody was used to immunoprecipitate p38 from rat brain lysate. Anti-mouse IgG was used as a negative control to assess nonspecific binding of the channel (lane 2). Immunoblot analysis of the IP complex was done with the p38 antibody. Anti-pan sodium channel antibody immunoprecipitated p38 from rat brain lysate (lane 3). D, MAPK p38 antibody was used to immunoprecipitate Nav1.6 from lysates of HEK293 cells transfected with either p38α (control; lanes 1, 3) or p38α plus Nav1.6 (lanes 2, 4). The IP complex was probed with the pan sodium channel antibody and detected no association between endogenous proteins with p38α (lane 3) but an association of Nav1.6 with p38α (lane 4). Lanes 1 and 2 show immunoblot analysis of the cell lysates probed with pan sodium channel antibody, demonstrating comparable levels of p38α (lane 1) and Nav1.6/p38α (lane 2) in the samples used for the IP assay. Immunoblot analysis using the anti-Flag antibody shows equal expression of the p38α protein (bottom). The lysate sample used as a positive control in lane 1 (B, C) and lanes 1 and 2 (D) is one-tenth that used in the immunoprecipitation assays. WB, Western blot.
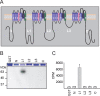
Figure 2.
The first intracellular loop of Nav1.6 is phosphorylated by p38α in an in vitro kinase assay. A, Schematic of Nav1.6 showing the intracellular regions that were used in the kinase assay. The N, L1, L2, L3, and C intracellular regions were produced as GST-fusion proteins. B, Autoradiographic film showing phosphorylation of the L1 region of Nav1.6 (top). The Coommassie blue-stained gel shows approximately equal expression of the GST-fusion proteins in the experiment. C, Cherenkov counts show the relative intensity of phosphorylation of GST, N, L1, L2, L3, and C.
Figure 3.
Deletion analysis of L1 delineates the site of p38 phosphorylation. Deletion derivatives of L1 were used to ascertain the site of p38 phosphorylation and the location of the MAPK recognition module. A, Schematic of the truncations made in GST-L1. L1 is the full-length GST-L1 protein that contains proximal (473-474) and distal (553-554) SP dipeptides. L1B contains both SP dipeptides but is missing the C-terminal half of L1. L1A contains only the proximal SP dipeptide and approximately the N-terminal one-fourth of L1. B, L1 and L1B are phosphorylated by p38α, but L1A is not phosphorylated. The Commassie blue-stained gel section of the figure shows equal amounts of the GST-fusion proteins used in this assay. C, Histogram of the average Cherenkov counts from the phosphorylation assays shows significant (*p < 0.01) phosphorylation of L1 and L1B compared with GST or L1A. L1B phosphorylation is significantly higher (**p < 0.01) than that of L1.
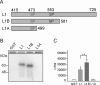
Figure 4.
Serine 553 is the phosphoacceptor site in L1 for p38 MAPK. A, Schematic of GST-L1B showing the substitution of the putative phosphoacceptor serine 553 by an alanine residue. B, Autoradiograph showing L1B phosphorylation but not L1Bmut. Even after 24 h of developing the autoradiograph, the phosphorylation of the L1Bmut is not detectable. The Commassie blue-stained fragments above the autoradiograph show a balanced amount of GST-fusion proteins used in each assay. C, Cherenkov counts from the phosphorylation experiment show significant (*p < 0.01) phosphorylation of L1B above GST or L1Bmut.
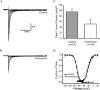
Figure 5.
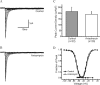
Anisomycin reduces peak current density of Nav1.6R. A, B, Representative I-V curve families of currents recorded in the presence of 0.25 μ
m
TTX are shown, in which cells were depolarized to a variety of potentials (-65 to +20 mV) from a holding potential of -120 mV to elicit Nav1.6R sodium current. Cells were treated with vehicle (DMSO) (A) or anisomycin (B) for 30 min before recording. C, Peak current density (in picoamperes per picofarads) of Nav1.6R currents showing a significant reduction with anisomycin treatment (*p < 0.05) compared with control. D, Availability and activation curves for Nav1.6R currents, showing no significant difference in measured biophysical properties.
Figure 6.
Reduction in peak current density by anisomycin is blocked by the specific p38 MAP kinase inhibitor SB203580. A-C, Representative I-V curve families of currents recorded in the presence of 0.25μ
m
TTX are shown, in which cells were depolarized to a variety of potentials (-65 to +20 mV) from a holding potential of -120 mV to elicit Nav1.6R sodium current. Cells were treated with the specific p38 MAP kinase inhibitor SB203580 for 60 min before recording (A), SB203580 for 60 min and anisomycin for 30 min (B), or the inactive analog SB202474 for 60 min and anisomycin for 30 min (C). D, Peak current density (in picoamperes per picofarads) of Nav1.6R currents, showing no significant effect of SB203580 alone (p > 0.05) and no reduction of current density with anisomycin treatment in the presence of SB203580 (p > 0.05). However, the reduction in current was still seen with anisomycin when cells were treated with the inactive analog SB202474 (*p < 0.05).
Figure 7.
Reduction in Nav1.6 peak current density by anisomycin is blocked by substituting serine 553 with alanine. A, B, Representative I-V curve families of currents recorded in the presence of 0.25 μ
m
TTX are shown, in which cells were depolarized to a variety of potentials (-65 to +20 mV) from a holding potential of -120 mV to elicit Nav1.6RSA current. Cells were treated with vehicle (DMSO) (A) or anisomycin (B) for 30 min before recording. C, Peak current density (in picoamperes per picofarads) of Nav1.6RSA currents showing no significant reduction with anisomycin treatment (NS) compared with control. D, Availability and activation curves for Nav1.6RSA currents, showing no significant difference in measured biophysical parameters.
Similar articles
- Phosphorylation of sodium channel Na(v)1.8 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons.
Hudmon A, Choi JS, Tyrrell L, Black JA, Rush AM, Waxman SG, Dib-Hajj SD. Hudmon A, et al. J Neurosci. 2008 Mar 19;28(12):3190-201. doi: 10.1523/JNEUROSCI.4403-07.2008. J Neurosci. 2008. PMID: 18354022 Free PMC article. - Two Nedd4-binding motifs underlie modulation of sodium channel Nav1.6 by p38 MAPK.
Gasser A, Cheng X, Gilmore ES, Tyrrell L, Waxman SG, Dib-Hajj SD. Gasser A, et al. J Biol Chem. 2010 Aug 20;285(34):26149-61. doi: 10.1074/jbc.M109.098681. Epub 2010 Jun 8. J Biol Chem. 2010. PMID: 20530479 Free PMC article. - Nav1.7-Ca2+ influx-induced increased phosphorylations of extracellular signal-regulated kinase (ERK) and p38 attenuate tau phosphorylation via glycogen synthase kinase-3beta: priming of Nav1.7 gating by ERK and p38.
Nemoto T, Miyazaki S, Kanai T, Maruta T, Satoh S, Yoshikawa N, Yanagita T, Wada A. Nemoto T, et al. Eur J Pharmacol. 2010 Aug 25;640(1-3):20-8. doi: 10.1016/j.ejphar.2010.04.048. Epub 2010 May 12. Eur J Pharmacol. 2010. PMID: 20470771 - Dynamic compartmentalization of the voltage-gated sodium channels in axons.
Garrido JJ, Fernandes F, Moussif A, Fache MP, Giraud P, Dargent B. Garrido JJ, et al. Biol Cell. 2003 Oct;95(7):437-45. doi: 10.1016/s0248-4900(03)00091-1. Biol Cell. 2003. PMID: 14597261 Review. - Mini-review - Sodium channels and beyond in peripheral nerve disease: Modulation by cytokines and their effector protein kinases.
Cheng X, Choi JS, Waxman SG, Dib-Hajj SD. Cheng X, et al. Neurosci Lett. 2021 Jan 10;741:135446. doi: 10.1016/j.neulet.2020.135446. Epub 2020 Nov 6. Neurosci Lett. 2021. PMID: 33166641 Review.
Cited by
- Apoptotic surge of potassium currents is mediated by p38 phosphorylation of Kv2.1.
Redman PT, He K, Hartnett KA, Jefferson BS, Hu L, Rosenberg PA, Levitan ES, Aizenman E. Redman PT, et al. Proc Natl Acad Sci U S A. 2007 Feb 27;104(9):3568-73. doi: 10.1073/pnas.0610159104. Epub 2007 Feb 20. Proc Natl Acad Sci U S A. 2007. PMID: 17360683 Free PMC article. - Downregulation of dendritic HCN channel gating in epilepsy is mediated by altered phosphorylation signaling.
Jung S, Bullis JB, Lau IH, Jones TD, Warner LN, Poolos NP. Jung S, et al. J Neurosci. 2010 May 12;30(19):6678-88. doi: 10.1523/JNEUROSCI.1290-10.2010. J Neurosci. 2010. PMID: 20463230 Free PMC article. - FGF14 N-terminal splice variants differentially modulate Nav1.2 and Nav1.6-encoded sodium channels.
Laezza F, Lampert A, Kozel MA, Gerber BR, Rush AM, Nerbonne JM, Waxman SG, Dib-Hajj SD, Ornitz DM. Laezza F, et al. Mol Cell Neurosci. 2009 Oct;42(2):90-101. doi: 10.1016/j.mcn.2009.05.007. Epub 2009 May 22. Mol Cell Neurosci. 2009. PMID: 19465131 Free PMC article. - Distinctive Properties and Powerful Neuromodulation of Nav1.6 Sodium Channels Regulates Neuronal Excitability.
Zybura A, Hudmon A, Cummins TR. Zybura A, et al. Cells. 2021 Jun 25;10(7):1595. doi: 10.3390/cells10071595. Cells. 2021. PMID: 34202119 Free PMC article. Review. - Tumor necrosis factor-α enhances voltage-gated Na⁺ currents in primary culture of mouse cortical neurons.
Chen W, Sheng J, Guo J, Gao F, Zhao X, Dai J, Wang G, Li K. Chen W, et al. J Neuroinflammation. 2015 Jun 26;12:126. doi: 10.1186/s12974-015-0349-x. J Neuroinflammation. 2015. PMID: 26112872 Free PMC article.
References
- Abriel H, Kamynina E, Horisberger JD, Staub O (2000) Regulation of the cardiac voltage-gated Na+ channel (H1) by the ubiquitin-protein ligase Nedd4. FEBS Lett 466: 377-380. - PubMed
- Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD (2000) The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem 75: 2277-2287. - PubMed
- Barone FC, Irving EA, Ray AM, Lee JC, Kassis S, Kumar S, Badger AM, White RF, McVey MJ, Legos JJ, Erhardt JA, Nelson AH, Ohlstein EH, Hunter AJ, Ward K, Smith BR, Adams JL, Parsons AA (2001) SB 239063, a second-generation p38 mitogen-activated protein kinase inhibitor, reduces brain injury and neurological deficits in cerebral focal ischemia. J Pharmacol Exp Ther 296: 312-321. - PubMed
- Bernard C, Anderson A, Becker A, Poolos NP, Beck H, Johnston D (2004) Acquired dendritic channelopathy in temporal lobe epilepsy. Science 305: 532-535. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases