An envelope-determined, pH-independent endocytic route of viral entry determines the susceptibility of human immunodeficiency virus type 1 (HIV-1) and HIV-2 to Lv2 restriction - PubMed (original) (raw)
An envelope-determined, pH-independent endocytic route of viral entry determines the susceptibility of human immunodeficiency virus type 1 (HIV-1) and HIV-2 to Lv2 restriction
David Marchant et al. J Virol. 2005 Aug.
Abstract
We identified a postentry restriction, termed Lv2, which determines the cellular tropism of two related human immunodeficiency virus type 2 (HIV-2) isolates and is dependent on the sequence of the capsid (CA) and envelope (Env) proteins. To explain the reliance on both CA and Env, we proposed that restrictive Envs deliver susceptible capsids to a compartment where Lv2 is active whereas nonrestrictive Envs deliver capsids into a compartment where Lv2 is either absent or less active. To test this model, we used compounds that affect endocytic pathways (ammonium chloride, bafilomycin A1, hypertonic sucrose) or lipid rafts (methyl-beta-cyclodextrin) to treat restrictive cells and show that restricted virus can be rescued from Lv2 if a lipid-raft-dependent, pH-independent endocytic pathway is inhibited. Furthermore, viral entry into HeLa/CD4 cells containing a tailless CD4 receptor, located outside lipid rafts, was fully permissive. Finally, we show that a variety of primary HIV-1 and HIV-2 viruses are susceptible to Lv2. Thus, we show that the route of entry, determined by the viral envelope, can influence cellular tropism by avoiding intracellular blocks to infection.
Figures
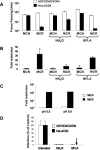
FIG. 1.
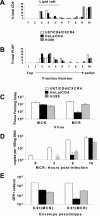
Ammonium chloride treatment of restrictive cells rescues MCR from Lv2 restriction; the viral envelope fusion is pH independent. (A) 200 FFU (on permissive U87/CD4/CXCR4) of either MCN or MCR HIV-2 virus were plated on restrictive HeLa/CD4 cells and the resulting FFU calculated (see Materials and Methods) in the presence or absence of 50 mM NH4Cl and 0.10 μM BFLA. (B) The fold restriction of each virus on HeLa/CD4 cells relative to that on U87/CD4/CXCR4 cells was calculated (see Materials and Methods). (C) MCR and MCN were titrated in acid medium pH 5.5 or physiological pH 6.8. (D) Both NH4Cl and BFLA treatments inhibit VSV-G-pseudotyped HIV-1-based vector. Vectors were titrated with or without NH4Cl or BFLA and GFP pplus vector cells quantitated by flow cytometry. Data is expressed as the number of GFP plus vector cells as a percentage of the untreated control. Error bars represent the standard errors of the mean from three independent experiments.
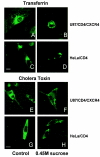
FIG. 2.
Sucrose inhibits transferrin and cholera toxin B uptake. trf-FITC (A through D) or CtxB-FITC (E through H) was bound to the permissive U87/CD4/CXCR4 or restrictive HeLa/CD4 cells with or without 0.45 M sucrose and examined by confocal microscopy. Inhibition is shown by trf-FITC or CtxB-FITC capping at the cell surface with simultaneous uptake into perinuclear (endosomal) regions of the cell. White bars indicate 10-μm magnification.
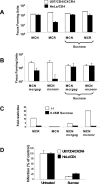
FIG. 3.
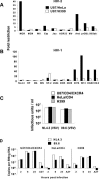
Endocytosis inhibition by hypertonic sucrose rescues MCR infection on HeLa/CD4. An equivalent input of 200 FFU (on U87/CD4/CXCR4 cells) of MCR and MCN (A) or MCNmcr_gag_ and MCNmcr_env_ gene swaps (B) was plated on restrictive HeLa/CD4 and nonrestrictive U87/CD4/CXCR4 cells with or without hypertonic sucrose. (C) The fold restriction was determined (from panels A and B, above) by dividing the infectious titer on U87/CD4/CXCR4 cells by the titer on HeLa/CD4 cells. (D) Sucrose treatment inhibits VSV-G-pseudotyped HIV-1-based vectors. Vector was titrated with or without sucrose and GFP plus vector cells were quantitated by flow cytometry. Data are expressed as the numbers of GFP plus vector cells as percentages of the untreated control. Error bars represent the standard errors of the mean from three independent experiments.
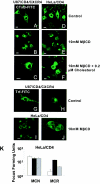
FIG.4.
Methyl-β-cyclodextrin differentially inhibits cholera toxin B uptake in restricted and nonrestricted cells. Permissive U87/CD4/CXCR4 or restrictive HeLa/CD4 cells were treated with MβCD. CtxB-FITC (A through F) and trf-FITC (G through J) were bound to treated or untreated cells for 1 h with or without water-soluble cholesterol and examined by confocal microscopy. Capping of CtxB-FITC at the cell surface shows inhibition. (K) MCN and MCR on HeLa/CD4 cells with or without MβCD treatment, with or without cholesterol replenishment. White bars denote no treatment, black bars denote MβCD, and grey bars denote replenishment with cholesterol.
FIG. 5.
CD4 membrane localization in permissive and restrictive cell lines: H399 CD4 localizes outside of lipid rafts. Lipid rafts were prepared from membrane lysates of U87/CD4/CXCR4, HeLa/CD4, and H399 and analyzed for CD4 (A) and PLAP localization (B) as a lipid raft marker. (C) An equivalent input of 200 FFU (on U87/CD4/CXCR4) of MCR and MCN was plated on U87/CD4/CXCR4, HeLa/CD4, and H399 and the FFUs calculated. (D) Time course of qPCR (strong-stop) of MCR on permissive and restrictive cell lines. (E) Vector pseudotypes were prepared from the MCN and MCR envelopes with HIV-1 gag-pol (p8.91) and titrated on permissive and restrictive cell lines. Transduced eGFP was measured by fluorescence-activated cell sorter and the number of eGFP-positive cells per ml calculated.
FIG. 6.
HIV-1 and HIV-2 isolates are susceptible to Lv2, rescued by the VSV-G envelope, and blocked post-reverse transcription. An array of HIV-2 (A) and HIV-1 (B) isolates was titrated on permissive U87/CD4/CXCR4, H399, and restrictive HeLa/CD4 cells and the FFUs calculated. Fold restriction was calculated by dividing the titer on permissive U87/CD4/CXCR4 cells by the titer on restrictive HeLa/CD4 cells or by permissive H399 cells. (C) VSV-G envelope pseudotypes were prepared with restricted HIV-1 89.6 or nonrestricted NL4.3 gag-pol and titrated on permissive and restrictive cell lines. Transduced eGFP was measured with a fluorescence-activated cell sorter and eGFP-positive cells per ml calculated. (D) Time course of qPCR, first-strand synthesis (ψ), of restricted HIV-1 89.6 and unrestricted HIV-1 NL4.3 on permissive and restrictive cell lines. Zidovudine (AZT) was added at 100 μM as a negative control for reverse transcription.
Similar articles
- The innate immune factor RPRD2/REAF and its role in the Lv2 restriction of HIV.
Jackson-Jones KA, McKnight Á, Sloan RD. Jackson-Jones KA, et al. mBio. 2023 Dec 19;14(6):e0257221. doi: 10.1128/mbio.02572-21. Epub 2023 Oct 26. mBio. 2023. PMID: 37882563 Free PMC article. Review. - Cellular entry via an actin and clathrin-dependent route is required for Lv2 restriction of HIV-2.
Harrison IP, McKnight A. Harrison IP, et al. Virology. 2011 Jun 20;415(1):47-55. doi: 10.1016/j.virol.2011.04.001. Epub 2011 Apr 22. Virology. 2011. PMID: 21514617 - Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4(+) T cells.
Popik W, Alce TM, Au WC. Popik W, et al. J Virol. 2002 May;76(10):4709-22. doi: 10.1128/jvi.76.10.4709-4722.2002. J Virol. 2002. PMID: 11967288 Free PMC article. - RNA-Associated Early-Stage Antiviral Factor Is a Major Component of Lv2 Restriction.
Marno KM, O'Sullivan E, Jones CE, Díaz-Delfín J, Pardieu C, Sloan RD, McKnight Á. Marno KM, et al. J Virol. 2017 Apr 28;91(10):e01228-16. doi: 10.1128/JVI.01228-16. Print 2017 May 15. J Virol. 2017. PMID: 28275184 Free PMC article. - Lipid rafts and HIV-1: from viral entry to assembly of progeny virions.
Campbell SM, Crowe SM, Mak J. Campbell SM, et al. J Clin Virol. 2001 Oct;22(3):217-27. doi: 10.1016/s1386-6532(01)00193-7. J Clin Virol. 2001. PMID: 11564586 Review.
Cited by
- Capsid-dependent lentiviral restrictions.
Twentyman J, Emerman M, Ohainle M. Twentyman J, et al. J Virol. 2024 Apr 16;98(4):e0030824. doi: 10.1128/jvi.00308-24. Epub 2024 Mar 18. J Virol. 2024. PMID: 38497663 Free PMC article. Review. - The innate immune factor RPRD2/REAF and its role in the Lv2 restriction of HIV.
Jackson-Jones KA, McKnight Á, Sloan RD. Jackson-Jones KA, et al. mBio. 2023 Dec 19;14(6):e0257221. doi: 10.1128/mbio.02572-21. Epub 2023 Oct 26. mBio. 2023. PMID: 37882563 Free PMC article. Review. - HIV-1 Accessory Protein Vpr Interacts with REAF/RPRD2 To Mitigate Its Antiviral Activity.
Gibbons JM, Marno KM, Pike R, Lee WJ, Jones CE, Ogunkolade BW, Pardieu C, Bryan A, Fu RM, Warnes G, Rowley PA, Sloan RD, McKnight Á. Gibbons JM, et al. J Virol. 2020 Jan 31;94(4):e01591-19. doi: 10.1128/JVI.01591-19. Print 2020 Jan 31. J Virol. 2020. PMID: 31776272 Free PMC article. - Inhibiting the Ins and Outs of HIV Replication: Cell-Intrinsic Antiretroviral Restrictions at the Plasma Membrane.
Foster TL, Pickering S, Neil SJD. Foster TL, et al. Front Immunol. 2018 Jan 4;8:1853. doi: 10.3389/fimmu.2017.01853. eCollection 2017. Front Immunol. 2018. PMID: 29354117 Free PMC article. Review. - A Virological and Phylogenetic Analysis of the Emergence of New Clades of Respiratory Syncytial Virus.
Elawar F, Griffiths CD, Zhu D, Bilawchuk LM, Jensen LD, Forss L, Tang J, Hazes B, Drews SJ, Marchant DJ. Elawar F, et al. Sci Rep. 2017 Sep 25;7(1):12232. doi: 10.1038/s41598-017-12001-6. Sci Rep. 2017. PMID: 28947776 Free PMC article.
References
- Bieniasz, P. D. 2004. Intrinsic immunity: a front-line defense against viral attack. Nat. Immunol. 5:1109-1115. - PubMed
- Clapham, P. R., and A. McKnight. 2002. Cell surface receptors, virus entry and tropism of primate lentiviruses. J. Gen. Virol. 83:1809-1829. - PubMed
- de Duve, C., T. de Barsy, B. Poole, A. Trouet, P. Tulkens, and F. Van Hoof. 1974. Commentary. Lysosomotropic agents. Biochem. Pharmacol. 23:2495-2531. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials