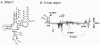Polyadenylation and degradation of human mitochondrial RNA: the prokaryotic past leaves its mark - PubMed (original) (raw)
Polyadenylation and degradation of human mitochondrial RNA: the prokaryotic past leaves its mark
Shimyn Slomovic et al. Mol Cell Biol. 2005 Aug.
Abstract
RNA polyadenylation serves a purpose in bacteria and organelles opposite from the role it plays in nuclear systems. The majority of nucleus-encoded transcripts are characterized by stable poly(A) tails at their mature 3' ends, which are essential for stabilization and translation initiation. In contrast, in bacteria, chloroplasts, and plant mitochondria, polyadenylation is a transient feature which promotes RNA degradation. Surprisingly, in spite of their prokaryotic origin, human mitochondrial transcripts possess stable 3'-end poly(A) tails, akin to nucleus-encoded mRNAs. Here we asked whether human mitochondria retain truncated and transiently polyadenylated transcripts in addition to stable 3'-end poly(A) tails, which would be consistent with the preservation of the largely ubiquitous polyadenylation-dependent RNA degradation mechanisms of bacteria and organelles. To this end, using both molecular and bioinformatic methods, we sought and revealed numerous examples of such molecules, dispersed throughout the mitochondrial genome. The broad distribution but low abundance of these polyadenylated truncated transcripts strongly suggests that polyadenylation-dependent RNA degradation occurs in human mitochondria. The coexistence of this system with stable 3'-end polyadenylation, despite their seemingly opposite effects, is so far unprecedented in bacteria and other organelles.
Figures
FIG. 1.
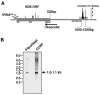
Analysis of truncated and polyadenylated COXI transcripts. A. Analysis using RT-PCR. The COXI transcript is schematically presented including the antisense region of tRNASer(UCN) (thin gray line). The primers used for PCR amplification of the oligo(dT)-primed cDNAs are shown by arrows below. The location of the oligonucleotide used for the RNase H-directed cleavage to remove the region containing the stable poly(A) tail is indicated by a horizontal black bar. Thin vertical lines indicate the positions of poly(A) tail addition. The dashed vertical line at the end of the COXI ORF designates an abundant but false polyadenylation position resulting from a gene-encoded poly(A) tract. Indeed, the poly(A) tails of these clones were shorter than 17 nt (see the supplemental material). Details concerning the polyadenylation positions and tail lengths are in the supplemental material. B. Analysis using PolyAfinder. COXI polyadenylation sites found in ESTs are presented as described for panel A. Of the 11 ESTs found at the end of the ORF, eight contained poly(A) tails longer than 17 adenosines. These were therefore considered authentic rather than resulting from annealing of the oligo(dT) primer to the A6 tract located nearby. C. RNA gel blot analysis of COXI. Total RNA was isolated from the cancer cell line CCRF-CEM and primary fibroblasts, and a gel blot was probed with a 32P-labeled double-stranded COXI probe. The migration of molecular size markers (in kilobases) is shown at the left. D. RNase protection assay to define the COXI mRNA 3′ end. Total RNA isolated from primary fibroblasts was annealed to a uniformly labeled probe of 342 nt spanning COXI, the antisense region of tRNASer(UCN), tRNAAsp, and several bases of COXII and ending with nine unrelated nucleotides. A parallel control reaction substituted an equivalent amount of yeast tRNA. The reaction mixtures were treated with RNase T1, and the protected fragments were analyzed by gel electrophoresis and autoradiography. The positions of RNA molecular size markers in nucleotides are shown at the left. The locations and sizes of the undigested probe and protected fragments are indicated to the right in the diagram below.
FIG. 2.
Polyadenylation of ND6 transcripts. A. Polyadenylation analysis using RT-PCR. The ND6 transcript from the first AUG to the translational termination codon is presented as a thick gray line ending at position 525. The downstream region is presented as a thin gray line, and tRNAGlu, located upstream of ND6, is indicated by a cloverleaf structure. Below the diagram, PCR primers are shown as gray arrows, and the antisense riboprobe is shown as a horizontal arrow. Thin vertical lines indicate the positions of poly(A) addition, with lengths proportional to the number of clones obtained. B. RNA gel blot analysis of ND6. Analysis was carried out as described in the legend to Fig. 1C, except that the probe was the uniformly labeled antisense RNA shown in panel A.
FIG. 3.
The heterogeneous 3′ termini of ND6 mRNA are located ∼500 nucleotides downstream of the translation termination codon. A. The ND6 region is schematically presented as described for Fig. 2A. Probe I (333 nt) is complementary to a segment of the ORF and the immediate downstream region and ends with 10 unrelated nucleotides. An ND6 transcript terminating at the stop codon would be expected to protect a 293-nt fragment; this product was not observed, and therefore the line representing it is crossed out. Probe II (334 nt) covers the downstream region in which poly(A) addition sites were located by RT-PCR. The full-length probe and the predicted protected products are presented. B. RNase protection analysis using probe I. Total RNA isolated from CCRF-CEM cancer cells was annealed with probe I and analyzed as described in the legend to Fig. 1D. The position where product of 293 nt would migrate is shown, and the positions of the observed 324-nt product and the full-length probe are indicated at the right. C. RNase protection analysis using probe II. Total RNA was isolated from three cancer cell lines (CCRF-CEM, CCRF-CEM/MTA, and MCF-7) as well as four negative controls including equal amounts of yeast tRNA, Arabidopsis thaliana chloroplasts, E. coli, and Haloferax volcanii RNAs. The migration of RNA markers is indicated at the left, and the probe and protected bands are shown at the right.
FIG. 4.
Polyadenylation of 16S rRNA. A. Analysis using RT-PCR. The transcript is presented schematically with the locations of PCR primers indicated by gray arrows. Thin vertical lines indicate the positions of poly(A) addition. B. Analysis using PolyAfinder. Sites of polyadenylation found in ESTs by PolyAfinder are presented as for panel A.
FIG. 5.
A. Polyadenylation of tRNALys. The positions of poly(A) addition and tail lengths as determined by RT-PCR are shown in gray. In cases in which polyadenylation occurred in close proximity to a DNA-encoded adenosine, the stated tail length includes this residue. The PCR primer is represented as a dashed gray arrow. B. Polyadenylation analysis of the D-loop region. The region between tRNAThr and tRNAPhe, which includes the D loop, is presented schematically. The positions of poly(A) addition revealed by the PolyAfinder are indicated by vertical lines above or below for the H and L strands, respectively. The length of each line is proportional to the number of ESTs found at that location. The transcription initiation sites on the H (ITH1 and ITH2) and L (ITL) strands as well as the origin of replication on the H strand (OH) are indicated. The tRNAs are represented by cloverleaf symbols using single-letter designations for the amino acids.
FIG. 6.
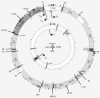
Analysis of the human EST database for polyadenylated mitochondrial transcripts. Poly(A) addition positions revealed by PolyAfinder are indicated by radial lines. The external and internal circles represent the H and L strands, respectively. Long lines represent sites for which >10 ESTs were found, with the precise number indicated beside the line. When several such sites are clustered, the number of sites is indicated in parentheses. The tRNA genes are indicated by single-letter symbols. The origins of replication and transcription initiation sites are denoted as in Fig. 5B.
Similar articles
- Detection and characterization of polyadenylated RNA in Eukarya, Bacteria, Archaea, and organelles.
Slomovic S, Portnoy V, Schuster G. Slomovic S, et al. Methods Enzymol. 2008;447:501-20. doi: 10.1016/S0076-6879(08)02224-6. Methods Enzymol. 2008. PMID: 19161858 - Polyadenylation of ribosomal RNA in human cells.
Slomovic S, Laufer D, Geiger D, Schuster G. Slomovic S, et al. Nucleic Acids Res. 2006 May 31;34(10):2966-75. doi: 10.1093/nar/gkl357. Print 2006. Nucleic Acids Res. 2006. PMID: 16738135 Free PMC article. - RNA polyadenylation and decay in mitochondria and chloroplasts.
Schuster G, Stern D. Schuster G, et al. Prog Mol Biol Transl Sci. 2009;85:393-422. doi: 10.1016/S0079-6603(08)00810-6. Prog Mol Biol Transl Sci. 2009. PMID: 19215778 Review. - Polyadenylation occurs at multiple sites in maize mitochondrial cox2 mRNA and is independent of editing status.
Lupold DS, Caoile AG, Stern DB. Lupold DS, et al. Plant Cell. 1999 Aug;11(8):1565-78. doi: 10.1105/tpc.11.8.1565. Plant Cell. 1999. PMID: 10449588 Free PMC article. - Exonucleases and endonucleases involved in polyadenylation-assisted RNA decay.
Slomovic S, Schuster G. Slomovic S, et al. Wiley Interdiscip Rev RNA. 2011 Jan-Feb;2(1):106-23. doi: 10.1002/wrna.45. Epub 2010 Sep 15. Wiley Interdiscip Rev RNA. 2011. PMID: 21956972 Review.
Cited by
- The process of mammalian mitochondrial protein synthesis.
Mai N, Chrzanowska-Lightowlers ZM, Lightowlers RN. Mai N, et al. Cell Tissue Res. 2017 Jan;367(1):5-20. doi: 10.1007/s00441-016-2456-0. Epub 2016 Jul 14. Cell Tissue Res. 2017. PMID: 27411691 Free PMC article. Review. - Common mitochondrial deletions in RNA-Seq: evaluation of bulk, single-cell, and spatial transcriptomic datasets.
Omidsalar AA, McCullough CG, Xu L, Boedijono S, Gerke D, Webb MG, Manojlovic Z, Sequeira A, Lew MF, Santorelli M, Serrano GE, Beach TG, Limon A, Vawter MP, Hjelm BE. Omidsalar AA, et al. Commun Biol. 2024 Feb 17;7(1):200. doi: 10.1038/s42003-024-05877-4. Commun Biol. 2024. PMID: 38368460 Free PMC article. - Panicum Mosaic Virus and Its Satellites Acquire RNA Modifications Associated with Host-Mediated Antiviral Degradation.
Pyle JD, Mandadi KK, Scholthof KBG. Pyle JD, et al. mBio. 2019 Aug 27;10(4):e01900-19. doi: 10.1128/mBio.01900-19. mBio. 2019. PMID: 31455653 Free PMC article. - Development of a human mitochondrial oligonucleotide microarray (h-MitoArray) and gene expression analysis of fibroblast cell lines from 13 patients with isolated F1Fo ATP synthase deficiency.
Cízková A, Stránecký V, Ivánek R, Hartmannová H, Nosková L, Piherová L, Tesarová M, Hansíková H, Honzík T, Zeman J, Divina P, Potocká A, Paul J, Sperl W, Mayr JA, Seneca S, Houstĕk J, Kmoch S. Cízková A, et al. BMC Genomics. 2008 Jan 25;9:38. doi: 10.1186/1471-2164-9-38. BMC Genomics. 2008. PMID: 18221507 Free PMC article. - Human mitochondrial ribosomes can switch structural tRNAs - but when and why?
Chrzanowska-Lightowlers Z, Rorbach J, Minczuk M. Chrzanowska-Lightowlers Z, et al. RNA Biol. 2017 Dec 2;14(12):1668-1671. doi: 10.1080/15476286.2017.1356551. Epub 2017 Sep 13. RNA Biol. 2017. PMID: 28786741 Free PMC article. Review.
References
- Bhat, K. S., N. K. Bhat, G. R. Kulkarni, A. Iyengar, and N. G. Avadhani. 1985. Expression of the cytochrome b-URF6-URF5 region of the mouse mitochondrial genome. Biochemistry 24:5818-5825. - PubMed
- Bollenbach, T. J., G. Schuster, and D. B. Stern. 2004. Cooperation of endo- and exoribonucleases in chloroplast mRNA turnover. Prog. Nucleic Acid Res. Mol. Biol. 78:305-337. - PubMed
- Carpousis, A. J., N. F. Vanzo, and L. C. Raynal. 1999. mRNA degradation, a tale of poly(A) and multiprotein machines. Trends Genet. 15:24-28. - PubMed
- Cheng, Z. F., and M. P. Deutscher. 2005. An important role for RNase R in mRNA decay. Mol. Cell 17:313-318. - PubMed
- Coburn, G. A., and G. A. Mackie. 1999. Degradation of mRNA in Escherichia coli: an old problem with some new twists. Prog. Nucleic Acid Res. 62:55-108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous