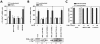Convergent actions of I kappa B kinase beta and protein kinase C delta modulate mRNA stability through phosphorylation of 14-3-3 beta complexed with tristetraprolin - PubMed (original) (raw)
Convergent actions of I kappa B kinase beta and protein kinase C delta modulate mRNA stability through phosphorylation of 14-3-3 beta complexed with tristetraprolin
Sonja I Gringhuis et al. Mol Cell Biol. 2005 Aug.
Abstract
Regulation of gene expression at the level of mRNA stability is a major topic of research; however, knowledge about the regulatory mechanisms affecting the binding and function of AU-rich element (ARE)-binding proteins (AUBPs) in response to extracellular signals is minimal. The beta1,4-galactosyltransferase 1 (beta4GalT1) gene enabled us to study the mechanisms involved in binding of tristetraprolin (TTP) as the stability of its mRNA is regulated solely through one ARE bound by TTP in resting human umbilical vein endothelial cells. Here, we provide evidence that the complex formation of TTP with 14-3-3beta is required to bind beta4GalT1 mRNA and promote its decay. Furthermore, upon tumor necrosis factor alpha stimulation, the activation of both Ikappabeta kinase and protein kinase Cdelta is involved in the phosphorylation of 14-3-3beta on two serine residues, paralleled by release of binding of TTP and 14-3-3beta from beta4GalT1 mRNA, nuclear sequestration of TTP, and beta4GalT1 mRNA stabilization. Thus, a key mechanism regulating mRNA binding and function of the destabilizing AUBP TTP involves the phosphorylation status of 14-3-3beta.
Figures
FIG. 1.
TTP binds to AU2 of β4GalT1 3′UTR, but is released upon TNF-α stimulation. (A) The expression of β4GalT1 mRNA is higher in HeLa TO cells that lack the expression of TTP but normal in HeLa TO cells lacking BRF1 expression. Stable HeLa TO knockdown cell lines for both TTP and BRF1 were generated as described in Materials and Methods; a scrambled (scr) control was included to monitor nonspecific effects. Quantitative real-time PCR analysis was performed on mRNA isolated from resting cells to determine the relative expression level of β4GalT1 mRNA. (B) The half-life of β4GalT1 mRNA is prolonged in TTP but not BRF1 knockdown HeLa TO cells. The half-life of β4GalT1 mRNA was determined by quantitative real-time PCR analysis of mRNA isolated from resting cells at different times after addition of ActD. (C) Overexpression of TTP accelerates the decay of chimeric mRNA containing the wild-type (wt) β4GalT1 3′UTR, but not the AU2 mutant. TTP was overexpressed in HeLa TO cells cotransfected to express chimeric mRNA containing either the wt β4GalT1 3′UTR or the AU2 mutant. At 24 h after transfection, the half-life of chimeric mRNAs was determined by quantitative real-time PCR analysis on mRNA isolated at different times after addition of doxycycline. The graphs (relative chimeric mRNA expression versus time after addition of DOX) for the determination of the half-lives are available in Fig. S1 in the supplemental material. The overexpression of His-tagged TTP was checked by Western blotting with an anti-His antibody as described in Materials and Methods. (D) TNF-α induces the release of binding of TTP from β4GalT1 mRNA. HUVECs, stimulated with 100 U/ml TNF-α as indicated, were treated with formaldehyde to preserve protein complexes bound to mRNA by reversible cross-links. Anti-TTP immunocomplexes were analyzed for the presence of β4GalT1 mRNA by quantitative real-time PCR. (E) TTP binds AU2. RNA/immunoprecipitation assays were performed on HeLa TO cells expressing chimeric mRNAs containing either the wt β4GalT1 3′UTR or the AU2 mutant as described in panel D.
FIG. 2.
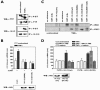
Activation of both IKKβ and PKCδ by TNF-α is required for β4GalT1 mRNA stabilization. (A) Inhibitors of IKK (BAY11-7082) and PKCδ (rottlerin) antagonize TNF-α-induced β4GalT1 mRNA stabilization, while the p38 inhibitor SB203580 has no effect. HUVECs were treated with various inhibitors as indicated, before the half-life of β4GalT1 mRNA was determined as described in the legend to Fig. 1B. The graphs (relative β4GalT1 mRNA expression versus time after the addition of ActD) for the determination of the half-lives are available in Fig. S2A in the supplemental material. (B) IKK activity is required but not sufficient to reduce the β4GalT1 mRNA turnover. Dominant-interfering [IKKγ(Δ246-365), IKKα(S176/180A), and IKKβ(S177/181A)], and kinase-active [IKKα(S176/180E), IKKβ(S177/181E)] mutants of IKK components were overexpressed in HeLa TO cells expressing chimeric mRNA containing the wt β4GalT1 3′UTR. Its half-life was determined as in the results shown in Fig. 1C. The graphs (relative chimeric mRNA expression versus time after addition of DOX) for the determination of the half-lives are available in Fig. S2B in the supplemental material. The overexpression of flag- or HA-tagged IKK mutants was checked by Western blotting with anti-flag and anti-HA antibodies as described in Materials and Methods. (C) Inhibitors of either IKK or PKCδ (BAY11-7082 and rottlerin, respectively) block the TNF-α-induced release of binding of TTP from β4GalT1 mRNA. RNA/immunoprecipitation assays were performed on HUVECs in either the absence or presence of inhibitors as described in the legend to Fig. 1D.
FIG. 3.
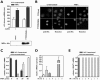
Nuclear sequestration of TTP induces β4GalT1 mRNA stabilization. (A) TNF-α-induced nuclear translocation of TTP is inhibited by inhibitors of IKK and PKCδ. HUVECs were grown in chamber slides, treated with inhibitors, and stimulated for 1 h with TNF-α as indicated. Subcellular localizaton of TTP was determined by immunofluorescence microscopy after staining with anti-TTP antibodies. Hoechst staining indicates the position of nuclei. (B) Inhibition of TTP nuclear export by LMB stabilizes β4GalT1 mRNA. HUVECs were treated with LMB, before the half-life of β4GalT1 mRNA was determined as described in the legend to Fig. 1B. The graphs (relative β4GalT1 mRNA expression versus time after addition of ActD) for the determination of the half-lives are available in Fig. S3 in the supplemental material.
FIG. 4.
Complex formation of TTP with 14-3-3β is required for and intact on mRNA binding. (A) A TTP mutant lacking the ability to complex with 14-3-3 has no effect on β4GalT1 mRNA stability. His-TTP(S186A) was overexpressed in HeLa TO cells expressing chimeric mRNA containing the wt β4GalT1 3′UTR. Its half-life was determined as described in the legend to Fig. 1C. The graphs (relative chimeric mRNA expression versus time after the addition of DOX) for the determination of the half-lives are available in Fig. S4 in the supplemental material. The overexpression of His-tagged TTP(S186A) was checked by Western blotting with an anti-His antibody as described in Materials and Methods. (B) TTP(S186A) is localized in the nucleus. HeLa TO cells expressing His-TTP(S186A) were grown in chamber slides. Subcellular localization of mutant TTP was determined by immunofluorescence microscopy after staining with anti-His antibodies. Hoechst staining indicates the position of nuclei. (C) TNF-α induces the dissociation of 14-3-3 from β4GalT1 mRNA. HUVECs, stimulated with TNF-α as indicated, were treated with formaldehyde to preserve protein complexes bound to mRNA by reversible cross-links. Anti-pan 14-3-3 immunocomplexes were analyzed for the presence of β4GalT1 mRNA by quantitative real-time PCR. (D) HUVECs express a subset of 14-3-3 isoforms. Quantitative real-time PCR analysis of 14-3-3 isoforms was performed on mRNA isolated from resting HUVECs to determine the relative expression levels. (E) 14-3-3β is the only isoform associated with β4GalT1 mRNA. RNA/immunoprecipitation assays were performed on HUVECs as described in the legend to Fig. 1D, using specific antibodies directed against the different isoforms instead of the anti-pan 14-3-3 antibodies.
FIG. 5.
Phosphorylation of 14-3-3β by IKKβ and PKCδ promotes β4GalT1 mRNA stabilization. (A) TNF-α stimulation induces serine/threonine phosphorylation of 14-3-3β, while TTP is already heavily phosphorylated on serine/threonine residues in resting HUVECs. Phospho-serine/threonine (P-S/T)-containing proteins were immunoprecipitated from lysates of unstimulated HUVECs or HUVECs stimulated for 15 min with TNF-α. Western blotting was performed using anti-TTP and anti-14-3-3β antibodies. Total levels of TTP and 14-3-3β were also checked by control immunoprecipitations. (B) 14-3-3β mutants carrying S60A or S132A substitutions interfere with TNF-α-induced β4GalT1 mRNA stabilization. 14-3-3β(S60A) or 14-3-3β(S132A) was overexpressed in HeLa TO cells expressing chimeric mRNA containing the wt β4GalT1 3′UTR. Its half-life was determined as described in the legend to Fig. 1C. The graphs (relative chimeric mRNA expression versus time after the addition of DOX) for the determination of the half-lives are available in Fig. S5A in the supplemental material. The overexpression of HA-tagged 14-3-3β(S60A) and 14-3-3β(S132A) was checked by Western blotting with an anti-HA antibody as described in Materials and Methods. (C) Ser60 and Ser132 of 14-3-3β are phosphorylated by PKCδ and IKKβ in vitro. PKCδ and IKKβ were immunoprecipitated from lysates of u nstimulated HUVECs or HUVECs stimulated for 15 min with TNF-α. Bacterially expressed GST-14-3-3β fusion proteins were used as substrates in kinase assays. (D) Acquired resistance to rottlerin in 14-3-3β(S132E)-expressing cells with regard to TNF-α-induced β4GalT1 mRNA stabilization. 14-3-3β(S132E) was overexpressed in HeLa TO cells expressing chimeric mRNA containing the wt β4GalT1 3′UTR. The cells were treated with BAY11-7082 or rottlerin as indicated, before the half-life of the chimeric mRNA was determined as described in the legend to Fig. 1C. The graphs (relative chimeric mRNA expression versus time after the addition of DOX) for the determination of the half-lives are available in Fig. S5B in the supplemental material. The overexpression of HA-tagged 14-3-3β(wt) and 14-3-3β(S132E) was checked by Western blotting with an anti-HA antibody as described in Materials and Methods.
Similar articles
- Structure/function analysis of tristetraprolin (TTP): p38 stress-activated protein kinase and lipopolysaccharide stimulation do not alter TTP function.
Rigby WF, Roy K, Collins J, Rigby S, Connolly JE, Bloch DB, Brooks SA. Rigby WF, et al. J Immunol. 2005 Jun 15;174(12):7883-93. doi: 10.4049/jimmunol.174.12.7883. J Immunol. 2005. PMID: 15944294 - Mitogen-activated protein kinase-activated protein kinase 2 regulates tumor necrosis factor mRNA stability and translation mainly by altering tristetraprolin expression, stability, and binding to adenine/uridine-rich element.
Hitti E, Iakovleva T, Brook M, Deppenmeier S, Gruber AD, Radzioch D, Clark AR, Blackshear PJ, Kotlyarov A, Gaestel M. Hitti E, et al. Mol Cell Biol. 2006 Mar;26(6):2399-407. doi: 10.1128/MCB.26.6.2399-2407.2006. Mol Cell Biol. 2006. PMID: 16508014 Free PMC article. - Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor alpha mRNA stability.
Mahtani KR, Brook M, Dean JL, Sully G, Saklatvala J, Clark AR. Mahtani KR, et al. Mol Cell Biol. 2001 Oct;21(19):6461-9. doi: 10.1128/MCB.21.9.6461-6469.2001. Mol Cell Biol. 2001. PMID: 11533235 Free PMC article. - Control of mRNA decay by phosphorylation of tristetraprolin.
Sandler H, Stoecklin G. Sandler H, et al. Biochem Soc Trans. 2008 Jun;36(Pt 3):491-6. doi: 10.1042/BST0360491. Biochem Soc Trans. 2008. PMID: 18481987 Review. - AU-rich element-mediated translational control: complexity and multiple activities of trans-activating factors.
Zhang T, Kruys V, Huez G, Gueydan C. Zhang T, et al. Biochem Soc Trans. 2002 Nov;30(Pt 6):952-8. doi: 10.1042/bst0300952. Biochem Soc Trans. 2002. PMID: 12440953 Review.
Cited by
- The role of tristetraprolin in cancer and inflammation.
Sanduja S, Blanco FF, Young LE, Kaza V, Dixon DA. Sanduja S, et al. Front Biosci (Landmark Ed). 2012 Jan 1;17(1):174-88. doi: 10.2741/3920. Front Biosci (Landmark Ed). 2012. PMID: 22201737 Free PMC article. Review. - The roles of TTP and BRF proteins in regulated mRNA decay.
Sanduja S, Blanco FF, Dixon DA. Sanduja S, et al. Wiley Interdiscip Rev RNA. 2011 Jan-Feb;2(1):42-57. doi: 10.1002/wrna.28. Wiley Interdiscip Rev RNA. 2011. PMID: 21278925 Free PMC article. Review. - Dual regulation of tumor necrosis factor-α on myosin light chain phosphorylation in vascular smooth muscle.
Chen M, Ma L, Hall JE, Liu X, Ying Z. Chen M, et al. Am J Physiol Heart Circ Physiol. 2015 Mar 1;308(5):H398-406. doi: 10.1152/ajpheart.00691.2014. Epub 2014 Dec 12. Am J Physiol Heart Circ Physiol. 2015. PMID: 25502110 Free PMC article. - Nuclear factor-kappa-B signaling in lung development and disease: one pathway, numerous functions.
Alvira CM. Alvira CM. Birth Defects Res A Clin Mol Teratol. 2014 Mar;100(3):202-16. doi: 10.1002/bdra.23233. Epub 2014 Mar 17. Birth Defects Res A Clin Mol Teratol. 2014. PMID: 24639404 Free PMC article. Review. - A novel function of Tis11b/BRF1 as a regulator of Dll4 mRNA 3'-end processing.
Desroches-Castan A, Cherradi N, Feige JJ, Ciais D. Desroches-Castan A, et al. Mol Biol Cell. 2011 Oct;22(19):3625-33. doi: 10.1091/mbc.E11-02-0149. Epub 2011 Aug 10. Mol Biol Cell. 2011. PMID: 21832157 Free PMC article.
References
- Aitken, A., H. Baxter, T. Dubois, S. Clokie, S. Mackie, K. Mitchell, A. Peden, and E. Zemlickova. 2002. Specificity of 14-3-3 isoform dimer interactions and phosphorylation. Biochem. Soc. Trans. 30:351-360. - PubMed
- Bevilacqua, A., M. C. Ceriani, S. Capaccioli, and A. Nicolin. 2003. Post-transcriptional regulation of gene expression by degradation of messenger RNAs. J. Cell. Physiol. 195:356-372. - PubMed
- Blackshear, P. J. 2002. Tristetraprolin and other CCCH tandem zinc-finger proteins in the regulation of mRNA turnover. Biochem. Soc. Trans. 30:945-952. - PubMed
- Brooks, S. A., J. E. Connolly, R. J. Diegel, R. A. Fava, and W. F. Rigby. 2002. Analysis of the function, expression, and subcellular distribution of human tristetraprolin. Arthritis Rheum. 46:1362-1370. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous