NF-kappa B-mediated MyoD decay during muscle wasting requires nitric oxide synthase mRNA stabilization, HuR protein, and nitric oxide release - PubMed (original) (raw)
NF-kappa B-mediated MyoD decay during muscle wasting requires nitric oxide synthase mRNA stabilization, HuR protein, and nitric oxide release
Sergio Di Marco et al. Mol Cell Biol. 2005 Aug.
Abstract
Muscle wasting (cachexia) is a consequence of chronic diseases, such as cancer, and is associated with degradation of muscle proteins such as MyoD. The cytokines tumor necrosis factor alpha and gamma interferon induce muscle degeneration by activating the transcription factor NF-kappaB and its target genes. Here, we show that a downstream target of NF-kappaB is the nitric oxide (NO) synthase gene (iNos) and suggest that NO production stimulates MyoD mRNA loss. In fact, although cytokine treatment of iNos(-/-) mice activated NF-kappaB, it did not trigger MyoD mRNA degeneration, demonstrating that NF-kappaB-mediated muscle wasting requires an active iNOS-NO pathway. The induced expression of iNOS by cytokines relies on both transcriptional activation via NF-kappaB and increased mRNA stability via the RNA-binding protein HuR. Moreover, we show that HuR regulates iNOS expression in an AMP-activated protein kinase (AMPK)-dependent manner. Furthermore, AMPK activation results in HuR nuclear sequestration, inhibition of iNOS synthesis, and reduction in cytokine-induced MyoD loss. These results define iNOS and HuR as critical players in cytokine-induced cachexia, establishing them as potential therapeutic targets.
Figures
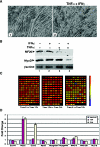
FIG. 1.
Microarray analysis of IFN-γ- and TNF-α-treated cells. Murine embryonic muscle C2C12 cells were induced for differentiation as soon as they reached 100% confluence and were treated 72 h later with 100 U/ml IFN-γ and 20 ng/ml TNF-α for 0, 12, and 24 h. RNAs extracted from these cells were then processed using the standard Affymetrix protocol, hybridized to Affymetrix Mouse Genome MU74Av2 oligonucleotide arrays, and analyzed qualitatively using Affymetrix GCOS software v1.2. The CHP files thus generated were then imported into GeneSpring v6.5 (Silicon Genetics) to identify changes in gene expression patterns. (A) Representative views of myofibers that were either untreated (panel 1) or treated with both IFN-γ and TNF-α (panel 2). (B) Western blot analysis of MyoD and the myosin heavy chain in myotubes treated with IFN-γ and/or TNF-α for 48 h or left untreated. (C) Computer-generated representation of genes showing a twofold or greater change in expression level at 12 h after IFN and TNF treatment (103 genes), 24 h after IFN and TNF treatment (162 genes) or between 12 and 24 h after IFN and TNF treatment (121 genes). Red bars represent increased expression of genes, while blue bars represent decreased gene expression. (D) Graphic representation of the differentially expressed genes observed at 12 and 24 h after IFN and TNF treatment. Nos2 is highly expressed in these treated muscle cells.
FIG. 2.
NO mediates the down-regulated expression of MyoD mRNA both in vitro and in vivo. (A) Total mRNA extracted from myotubes at various time points (0, 12, 24, 36 h) after treatment with or without IFN-γ and/or TNF-α were analyzed by Northern blot analysis using 32P-labeled cDNA probes to detect MyoD, iNOS, Myogenin, HuR, p21, and GAPDH mRNAs. (B) C2C12 myotubes were treated with or without IFN-γ (100 U/ml) and TNF-α (20 ng/ml) as well as 2-aminoguanidine (an iNOS inhibitor) for 24 h. NO quantification was performed as described in Materials and Methods. (C) Untreated (−) as well as IFN-γ (I)- and TNF-α (T)-treated myotubes (IT) (panel 4) were incubated with or without 2-aminoguanidine (AMG) or FeTPPS for 24 h. (D) Total RNA was prepared from both untreated as well as IFN-γ- and TNF-α-treated C2C12 myotubes with or without AMG or FeTPPS for 24 h. MyoD and iNOS mRNA levels were then analyzed by Northern blot. GAPDH mRNA levels are included as a loading control for Northern blots in panels A and D. (E) The gastrocnemius muscle was from wild-type (iNOS+/+) and homozygote (iNOS−/−) mice, which were injected twice (every 8 h) with the IFN-γ and TNF-α or with saline for 24 h. Total mRNAs from all treated muscles were prepared and analyzed by Northern blotting to monitor the expression of iNOS, MuRf1, and MyoD mRNAs.
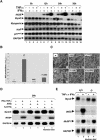
FIG. 3.
C2C12 myotubes overexpressing a NF-κB suppressor (IκB) are protected against cytokine- but not medium starvation-induced atrophy, despite the absence of iNOS expression under both conditions. C2C12 myotubes stably expressing either a NF-κB suppressor (HcNeoSR or IFN-γ [I]) or an empty vector (HcNeo or V) were induced for differentiation and then either treated with IFN-γ and TNF-α for various periods of time (0, 6, 12, and 24 h) (panels A to C and F) or medium starved for 6 h (panels D and E). C2C12 myotubes (C2C12 or C) were included in the experiments as a positive control. (A) Total RNAs were collected and used for Northern blot analysis to follow iNOS mRNA expression. (B) NO release was quantified using GREISS reagent to determine the absorbance at 543 nm of the culture media obtained from the above-mentioned cells. (C) Total extract from the cells whose results are shown in panel B were analyzed by Western blotting using anti-iNOS, -tubulin, -IκBα, and -HuR antibodies in order to determine the expression of these proteins in the cells. Tubulin protein levels are included as a loading control. The expression of the transfected human IκΒα is visible due to the differential molecular weight of the endogenous (mouse) and the exogenous (human) IκΒα protein. (D and E) The same cells as described above were induced for differentiation for 72 h and than starved by removal of growth medium and incubated in phosphate-buffered saline for 6 h. (D) Total mRNAs were collected and analyzed by Northern blotting to assess iNOS mRNA expression. (E) The effect of medium starvation on differentiated myotubes is shown using the three C2C12 cell types described above. (F) Total mRNAs were harvested from differentiated HcNeo and HcNeoSR C2C12 cells treated for 0 h, 12 h, and 24 h with TNF-α and IFN-γ, and RT-PCR was performed using _MyoD_- and _GAPDH_-specific primers.
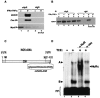
FIG. 4.
HuR associates with iNOS mRNA through an AU-rich element located in its 3′UTR. Myotubes were treated as described for Fig. 1, and total extracts were collected. (A and B) Immunoprecipitation experiments (B) using the anti-HuR antibody on total extracts from myotubes treated or not with cytokines for 24 h were followed by RT-PCR (A) in order to monitor HuR's association with iNOS, COX-2, and MyoD mRNAs. An anti-IgG antibody was used as a negative control. (B) A fraction (1/10) of the immunoprecipitated (P) or the flow-through (S) fraction was loaded on an 12% SDS-PAGE gel for Western blot analysis. Upon transfer, the membrane was probed with the anti-HuR monoclonal antibody. (C) Schematic representation of the mouse iNOS cDNA. The location, as well as the exact sequence, of putative AREs (miNOS-ARE) is included. (D) Gel-shift binding assays were performed by incubating total cell extract (TCE) from differentiating C2C12 cells (day 3) with a radiolabeled iNOS-ARE. Two complexes, A and B, were observed as a shift on a nondenaturing gel (4%) (lane 2) compared to a free probe alone (FP, lane 1). The complex containing HuR was detected by incubating TCE with the monoclonal anti-HuR antibody (α HuR). HuRc is the shifted complex upon addition of the anti-HuR antibody. The antivimentin antibody (α vim) was included in the assay as a negative control (lane 4).
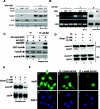
FIG. 5.
The cytokine-dependent expression of iNOS mRNA requires normal cellular levels of both HuR protein and ATP. (A) Protein extracts from mock (−)- and HuSi1 (siRNA duplexes against HuR as described previously) (56)- and control (CTL) siRNA-treated myoblasts (with or without IFN-γ and TNF-α treatment for 24 h) were analyzed by Western blotting using a monoclonal anti-HuR antibody or a anti-iNOS monoclonal antibody. β-Actin levels are included as a loading control. (B) Total RNAs from the above-described treated cells were analyzed by RT-PCR using _iNOS_- and _GAPDH_-specific primers. The same total mRNAs were used to perform Northern blot analysis to detect iNOS and GAPDH mRNA expression (lanes 7 and 8). (C) Mock (−)- or HuSi1 siRNA-transfected myoblasts were incubated with AP-HuR-GST or AP-GST (as control) 8 h prior to treatment with or without IFN-γ and TNF-α for an additional 24 h. iNOS and HuR protein levels were then assessed as described for panel A. (D to F) C2C12 cells were treated with IFN-γ and TNF-α and then stimulated with or without 2 or 4 mM AICAR for 24 h. (D) HuR protein levels were then analyzed by Western blotting in nuclear (NF) and cytoplasmic (Cyt) extracts. Actin protein levels are included as loading controls. (E) Northern blot experiments were performed with RNA extracted from C2Cl2 cells 12 h after IFN-γ and TNF-α treatment with or without 2 and 4 mM AICAR. (F) Cells were treated as indicated in panel D, and the localization of endogenous HuR was determined by double immunofluorescence staining using the anti-HuR antibody. DAPI- and HuR (fluorescein isothiocyanate)-stained images of a single representative field are shown.
FIG. 6.
The stability of iNOS mRNA as well as NO release is increased in C2C12 cells. (A) Quantification of NO levels in media collected from cells treated with or without IFN-γ and/or TNF-α for the indicated periods of time. (B) Myotubes were treated with IFN-γ and/or TNF-α for 12 h prior to being incubated with 5 μg/ml of actinomycin D (ActD) for the indicated time points. Total RNA were prepared and detected by Northern blotting as described for Fig. 2A. Endogenous GAPDH mRNA was used as loading control. (C) The expression of iNOS mRNA was quantified as described using the ImageQuant software program. Levels were then standardized against GAPDH levels and plotted as the percentage of remaining mRNA compared to message levels at the 0 time point (where there is a 100% maximum mRNA level). The half-lives of the mRNAs (where mRNA levels decrease to 50% of the maximum levels at t = 0) are indicated on the graph.
FIG. 7.
Model depicting the role of HuR-regulated iNOS mRNA and thus NO secretion in changes in MyoD mRNA levels. In muscle cells TNF-α and IFN-γ stimulate, respectively, the transcription factor NF-κB as well as IFN-γ-dependent transcription factors (such as STAT1), which in conjunction induce the mRNA expression of the iNos gene (34). The RNA-binding protein HuR, which is localized in the nucleus, associates with the iNOS mRNA through its ARE mediating its stability and probably its export to the cytoplasm. iNOS enzyme will likely induce NO conjugation with the superoxide (O2−) to form peroxynitrite. The release of OONO− either inside the cytoplasm or outside the cell will activate the down regulation process of MyoD mRNA. The exact mechanism leading to NO-dependent MyoD loss is still unclear and could be due to destabilization and decay of the message in the cytoplasm or to transcription inhibition of myod gene in the nucleus.
References
- Aktan, F. 2004. iNOS-mediated nitric oxide production and its regulation. Life Sci. 75:639-653. - PubMed
- Antic, D., and J. D. Keene. 1998. Messenger ribonucleoprotein complexes containing human ELAV proteins: interactions with cytoskeleton and translational apparatus. J. Cell Sci. 111:183-197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous