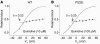Human cardiac potassium channel DNA polymorphism modulates access to drug-binding site and causes drug resistance - PubMed (original) (raw)
. 2005 Aug;115(8):2209-13.
doi: 10.1172/JCI23741. Epub 2005 Jul 14.
Affiliations
- PMID: 16025157
- PMCID: PMC1174915
- DOI: 10.1172/JCI23741
Human cardiac potassium channel DNA polymorphism modulates access to drug-binding site and causes drug resistance
Benoit Drolet et al. J Clin Invest. 2005 Aug.
Abstract
Expression of voltage-gated K channel, shaker-related subfamily, member 5 (KCNA5) underlies the human atrial ultra-rapid delayed rectifier K current (I(Kur)). The KCNA5 polymorphism resulting in P532L in the C terminus generates I(Kur) that is indistinguishable from wild type at baseline but strikingly resistant to drug block. In the present study, truncating the C terminus of KCNA5 generated a channel with wild-type drug sensitivity, which indicated that P532 is not a drug-binding site. Secondary structure prediction algorithms identified a probable alpha-helix in P532L that is absent in wild-type channels. We therefore assessed drug sensitivity of I(Kur) generated in vitro in CHO and HEK cells by channels predicted to exhibit or lack this C-terminal alpha-helix. All constructs displayed near-identical I(Kur) in the absence of drug challenge. However, those predicted to lack the C-terminal alpha-helix generated quinidine-sensitive currents (43-51% block by 10 microM quinidine), while the currents generated by those constructs predicted to generate a C-terminal alpha-helix were inhibited less than 12%. Circular dichroism spectroscopy revealed an alpha-helical signature with peptides derived from drug-resistant channels and no organized structure in those associated with wild-type drug sensitivity. In conclusion, we found that this secondary structure in the KCNA5 C terminus, absent in wild-type channels but generated by a naturally occurring DNA polymorphism, does not alter baseline currents but renders the channel drug resistant. Our data support a model in which this structure impairs access of the drug to a pore-binding site.
Figures
Figure 1
Electrophysiology and drug sensitivity of wild type (left panels) and P532L (right panels). (A) Wild-type activating current in CHO cells at +50 mV and tail current at –30 mV at baseline and after a 20-minute exposure to quinidine 10 μM at 37°C. (B) P532L studied under the same conditions as described for A. (C) Wild-type activating current in HEK cells at +50 mV and tail current at –30 mV at baseline and after a 20-minute exposure to quinidine 10 μM at room temperature. (D) P532L studied under the same conditions as described for C.
Figure 2
Calculation of δ. (A) Voltage dependence of wild-type KCNA5-mediated current inhibition by quinidine 10 μM (near the IC50). Current at the end of voltage steps in the presence of drug (_I_Drug) was normalized to matching control current (_I_Control) and converted to normalized block (1 – _I_Drug/_I_Control). Below –20 mV, the ratio was undefined (small or no current). The dotted line represents the voltage dependence of Kv1.5 activation. Vm indicates membrane voltage; open symbols represent steep voltage dependence of block coinciding with channel activation, and closed symbols, shallow voltage dependence of block. Only the latter data were used in the fit with the Woodhull model (see Methods), shown by the solid line with the indicated values for the equivalent δ. (B) The same experiment as shown in A, with P532L studied at a concentration near the IC50 (100 μM).
Figure 3
Electrophysiology and drug sensitivity of KCNA5 variants. (A) Current generated by ΔC-term527. The current displays wild-type sensitivity to drug. (B–D) P532E, P532M, and P532S, displaying resistance to drug block.
Figure 4
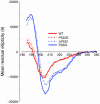
CD spectra from 185 to 260 nm in 2,2,2-trifluoroethanol, 40% vol/vol, at 25°C. The red traces represent data obtained using peptides lacking structural organization (solid line, wild type; dashed line, P532G). The blue traces were derived using peptides predicted to generate α-helices (solid line, P532L; dashed line, ΔP532, in which the proline at position 532 is deleted without substitution). The blue traces display minima at 208 and 222 nm, typical of α-helices.
Figure 5
Schematic representation of KCNA5 with its intracellular S6 drug binding site, indicated by arrows. The right panel illustrates the concept that generation of a C-terminal α-helical secondary structure in the P532L variant (black dot) would restrict access of the drug to the S6 binding site.
Similar articles
- Polymorphism screening in the cardiac K+ channel gene KCNA5.
Simard C, Drolet B, Yang P, Kim RB, Roden DM. Simard C, et al. Clin Pharmacol Ther. 2005 Mar;77(3):138-44. doi: 10.1016/j.clpt.2004.10.008. Clin Pharmacol Ther. 2005. PMID: 15735608 - Four and a half LIM protein 1: a partner for KCNA5 in human atrium.
Yang Z, Browning CF, Hallaq H, Yermalitskaya L, Esker J, Hall MR, Link AJ, Ham AJ, McGrath MJ, Mitchell CA, Murray KT. Yang Z, et al. Cardiovasc Res. 2008 Jun 1;78(3):449-57. doi: 10.1093/cvr/cvn038. Epub 2008 Feb 15. Cardiovasc Res. 2008. PMID: 18281375 - Modulation of drug block of the cardiac potassium channel KCNA5 by the drug transporters OCTN1 and MDR1.
Yang T, McBride BF, Leake BF, Kim RB, Roden DM. Yang T, et al. Br J Pharmacol. 2010 Nov;161(5):1023-33. doi: 10.1111/j.1476-5381.2010.00932.x. Br J Pharmacol. 2010. PMID: 20977453 Free PMC article. - Phosphorylation of the IKs channel complex inhibits drug block: novel mechanism underlying variable antiarrhythmic drug actions.
Yang T, Kanki H, Roden DM. Yang T, et al. Circulation. 2003 Jul 15;108(2):132-4. doi: 10.1161/01.CIR.0000082708.86266.B8. Epub 2003 Jun 30. Circulation. 2003. PMID: 12835205 - Ultra-rapid delayed rectifier channels: molecular basis and therapeutic implications.
Ravens U, Wettwer E. Ravens U, et al. Cardiovasc Res. 2011 Mar 1;89(4):776-85. doi: 10.1093/cvr/cvq398. Epub 2010 Dec 15. Cardiovasc Res. 2011. PMID: 21159668 Review.
Cited by
- The pharmacogenetics research network: from SNP discovery to clinical drug response.
Giacomini KM, Brett CM, Altman RB, Benowitz NL, Dolan ME, Flockhart DA, Johnson JA, Hayes DF, Klein T, Krauss RM, Kroetz DL, McLeod HL, Nguyen AT, Ratain MJ, Relling MV, Reus V, Roden DM, Schaefer CA, Shuldiner AR, Skaar T, Tantisira K, Tyndale RF, Wang L, Weinshilboum RM, Weiss ST, Zineh I; Pharmacogenetics Research Network. Giacomini KM, et al. Clin Pharmacol Ther. 2007 Mar;81(3):328-45. doi: 10.1038/sj.clpt.6100087. Clin Pharmacol Ther. 2007. PMID: 17339863 Free PMC article. Review. - Pharmacogenetic treatments for drug addiction: cocaine, amphetamine and methamphetamine.
Haile CN, Kosten TR, Kosten TA. Haile CN, et al. Am J Drug Alcohol Abuse. 2009;35(3):161-77. doi: 10.1080/00952990902825447. Am J Drug Alcohol Abuse. 2009. PMID: 19462300 Free PMC article. Review. - Electrophysiological characterization of three non-synonymous single nucleotide polymorphisms (R87Q, A251T, and P307S) found in hKv1.5.
Plante I, Fournier D, Ricard G, Drolet B, O'Hara G, Champagne J, Mathieu P, Baillot R, Daleau P. Plante I, et al. Pflugers Arch. 2006 Jun;452(3):316-23. doi: 10.1007/s00424-005-0031-8. Epub 2006 Jan 13. Pflugers Arch. 2006. PMID: 16411137 - Pharmacogenomics: challenges and opportunities.
Roden DM, Altman RB, Benowitz NL, Flockhart DA, Giacomini KM, Johnson JA, Krauss RM, McLeod HL, Ratain MJ, Relling MV, Ring HZ, Shuldiner AR, Weinshilboum RM, Weiss ST; Pharmacogenetics Research Network. Roden DM, et al. Ann Intern Med. 2006 Nov 21;145(10):749-57. doi: 10.7326/0003-4819-145-10-200611210-00007. Ann Intern Med. 2006. PMID: 17116919 Free PMC article. Review. - Novel molecular targets for atrial fibrillation therapy.
Dobrev D, Carlsson L, Nattel S. Dobrev D, et al. Nat Rev Drug Discov. 2012 Mar 30;11(4):275-91. doi: 10.1038/nrd3682. Nat Rev Drug Discov. 2012. PMID: 22460122 Review.
References
- Tamkun MM, et al. Molecular cloning and characterization of two voltage-gated K+ channel cDNAs from human ventricle. FASEB J. 1991;5:331–337. - PubMed
- Fedida D, et al. Identity of a novel delayed rectifier current from human heart with a cloned K+ channel current. Circ. Res. 1993;73:210–216. - PubMed
- Nattel S. New ideas about atrial fibrillation 50 years on [review] Nature. 2002;415:219–226. - PubMed
- Roden DM. Pharmacogenetics and drug-induced arrhythmias. Cardiovasc. Res. 2001;50:224–231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous