Activation of beta-catenin by carcinogenic Helicobacter pylori - PubMed (original) (raw)
Comparative Study
. 2005 Jul 26;102(30):10646-51.
doi: 10.1073/pnas.0504927102. Epub 2005 Jul 18.
Dawn A Israel, Mary K Washington, Uma Krishna, James G Fox, Arlin B Rogers, Andrew S Neish, Lauren Collier-Hyams, Guillermo I Perez-Perez, Masanori Hatakeyama, Robert Whitehead, Kristin Gaus, Daniel P O'Brien, Judith Romero-Gallo, Richard M Peek Jr
Affiliations
- PMID: 16027366
- PMCID: PMC1180811
- DOI: 10.1073/pnas.0504927102
Comparative Study
Activation of beta-catenin by carcinogenic Helicobacter pylori
Aime T Franco et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Persistent gastritis induced by Helicobacter pylori is the strongest known risk factor for adenocarcinoma of the distal stomach, yet only a fraction of colonized persons ever develop gastric cancer. The H. pylori cytotoxin-associated gene (cag) pathogenicity island encodes a type IV secretion system that delivers the bacterial effector CagA into host cells after bacterial attachment, and cag+ strains augment gastric cancer risk. A host effector that is aberrantly activated in gastric cancer precursor lesions is beta-catenin, and activation of beta-catenin leads to targeted transcriptional up-regulation of genes implicated in carcinogenesis. We report that in vivo adaptation endowed an H. pylori strain with the ability to rapidly and reproducibly induce gastric dysplasia and adenocarcinoma in a rodent model of gastritis. Compared with its parental noncarcinogenic isolate, the oncogenic H. pylori strain selectively activates beta-catenin in model gastric epithelia, which is dependent on translocation of CagA into host epithelial cells. Beta-catenin nuclear accumulation is increased in gastric epithelium harvested from gerbils infected with the H. pylori carcinogenic strain as well as from persons carrying cag+ vs. cag- strains or uninfected persons. These results indicate that H. pylori-induced dysregulation of beta-catenin-dependent pathways may explain in part the augmentation in the risk of gastric cancer conferred by this pathogen.
Figures
Fig. 1.
Development of premalignant and malignant lesions within gerbil gastric mucosa after infection with H. pylori strain 7.13. No significant pathologic abnormalities were present in gerbils challenged with broth alone (A). Dysplastic foci (B) were often accompanied by gastric carcinomas arising within a background of inflammation (C and D). Gastric carcinomas were characterized by marked cellular pleomorphism, cellular atypia, and euchromatic nuclei that exhibited bizarre morphologic features.
Fig. 2.
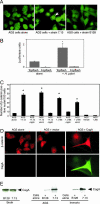
H. pylori strain 7.13 induces nuclear translocation of β-catenin in a CagA-dependent manner and activates a β-catenin responsive transcriptional reporter. (A) AGS gastric epithelial cells incubated with medium alone, strain 7.13, or strain B128 for 6 h were immunostained with an anti-β-catenin antibody and visualized by fluorescence microscopy. Representative images are shown. H. pylori strain 7.13, but not B128, induced β-catenin translocation to the nucleus (arrow). (B) AGS cells were transfected with luciferase reporter constructs containing LEF/TCF-binding motifs (Topflash) or mutated LEF/TCF sites (Fopflash) in the absence or presence of strain 7.13. Luciferase activity was determined after 24 h of treatment. *, P < 0.05 vs. Topflash alone. (C) AGS cells were cultured in the absence or presence of H. pylori cag+ strain 7.13 or isogenic cagA, vacA, or cagE mutant derivatives, progenitor strain B128, cag+ strain J166 or isogenic _cagA_- or _vacA_- mutants, or a _cag_- clinical strain (J68) for 6 h. The number of cells with nuclear β-catenin per 100 cells evaluated is shown for each sample. Error bars = SD. *, P < 0.05 vs. AGS cells alone. (D) Cellular distribution of β-catenin and CagA in AGS cells was detected by immunofluorescence (β-catenin, red; CagA, green) after transfection with reagent alone, vector alone, or wild-type CagA. (E) Lysates from H. pylori strains 7.13 or B128 grown in broth alone or after coculture with AGS or conditionally immortalized (Immorto) gastric epithelial cells were used for Western blot analysis using anti-CagA antibodies.
Fig. 3.
β-Catenin membrane localization is decreased within gerbil gastric mucosa early after infection with H. pylori strain 7.13. Immunohistochemistry for β-catenin was performed on gastric mucosa harvested from H. pylori strain 7.13-infected and uninfected gerbils euthanized 4 h, 24 h, and 1 week after inoculation. Representative staining for β-catenin is shown for uninfected (A, C, and E) and _H. pylori_-infected (B, D, and F) gerbils at the indicated time points. Arrows indicate cells with decreased β-catenin membrane staining and/or increased cytoplasmic and nuclear β-catenin.
Fig. 4.
Nuclear β-catenin is increased in gastric epithelium harvested from H. pylori cag+-colonized persons. (A) Gastric epithelial cells with nuclear β-catenin, as assessed by immunohistochemistry, were quantified by an observer unaware of H. pylori status. Results are expressed as the number of cells per sample with detectable nuclear β-catenin. Mean values (▴) are shown adjacent to data points (▪). Representative staining for β-catenin is shown for uninfected (B), _H. pylori cag_--infected (C), or H. pylori cag+-infected (D) persons. Arrows indicate nuclear β-catenin.
References
- Parsonnet, J., Friedman, G. D., Vandersteen, D. P., Chang, Y., Vogelman, J. H., Orentreich, N. & Sibley, R. K. (1991) N. Engl. J. Med. 325, 1127-1131. - PubMed
- Peek, R. M., Jr., & Blaser, M. J. (2002) Nat. Rev. Cancer 2, 28-37. - PubMed
- Odenbreit, S., Puls, J., Sedlmaier, B., Gerland, E., Fischer, W. & Haas, R. (2000) Science 287, 1497-1500. - PubMed
- Selbach, M., Moese, S., Hauck, C. R., Meyer, T. F. & Backert, S. (2002) J. Biol. Chem. 277, 6775-6778. - PubMed
- Higashi, H., Tsutsumi, R., Muto, S., Sugiyama, T., Azuma, T., Asaka, M. & Hatakeyama, M. (2002) Science 295, 683-686. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA077955/CA/NCI NIH HHS/United States
- R29 CA077955/CA/NCI NIH HHS/United States
- DK-58587/DK/NIDDK NIH HHS/United States
- CA-77955/CA/NCI NIH HHS/United States
- T32 CA009592/CA/NCI NIH HHS/United States
- R01 DK058587/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases