The promise and potential challenges of intermittent preventive treatment for malaria in infants (IPTi) - PubMed (original) (raw)
Review
The promise and potential challenges of intermittent preventive treatment for malaria in infants (IPTi)
Wendy Prudhomme O'Meara et al. Malar J. 2005.
Abstract
Intermittent preventive treatment (IPT) administers a full therapeutic course of an anti-malarial drug at predetermined intervals, regardless of infection or disease status. It is recommended by the World Health Organization (WHO) for protecting pregnant women from the adverse effects of malaria (IPTp) and shows great potential as a strategy for reducing illness from malaria during infancy (IPTi). Administered concurrently with standard immunizations, IPTi is expected to reduce the frequency of clinical disease, but to allow blood-stage infections to occur between treatments, thus allowing parasite-specific immunity to develop. While wide deployment of IPTi is being considered, it is important to assess other potential effects. Transmission conditions, drug choice and administration schedule will likely affect the possibility of post-treatment rebound in child morbidity and mortality and the increased spread of parasite drug resistance and should be considered when implementing IPTi.
Figures
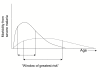
Figure 1
Concentration of anti-malarial drug in bloodstream during IPT. Drug concentration initially rises after administration of a therapeutic drug regimen. The concentration declines gradually as the drug is cleared from the bloodstream. Parasites with increasing sensitivity to the drug will be able to grow as the drug concentration declines. Parasites are divided roughly into three categories; fully sensitive, partially resistant and fully resistant. The interval between the time at which the concentration falls below the threshold for partially sensitive parasites and the time at which it falls below the threshold for sensitive parasites is a window of selection for resistant parasites. The dotted curve represents the target blood concentrations during chemoprophylaxis.
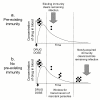
Figure 2
Effect of transmission intensity on outcome of IPTi. Qualitative depiction of the effect of transmission intensity on the age-incidence pattern of malaria morbidity and mortality. In areas of high transmission (solid line), malaria mortality is concentrated in the first two years of life. In areas of low or unstable transmission (dashed line), children may be at risk until much later in life. The arrows represent the window of greatest risk or the age interval during which roughly 75% of childhood malaria episodes are experienced.
Figure 3
Effect of transmission intensity on outcome of IPTi. Effect of biting frequency (arrows represent infectious bites) on the development of malaria-specific immunity during IPTi. The frequency of infectious bites determines whether or not an infant is exposed to malaria parasites between doses of drug during IPTi.
Figure 4
Drug treatment and immune mechanisms act synergistically to eliminate parasites. (a) A patient with some pre-existing immunity clears infection and reduces the number and transmission of the parasites that survive treatment. (b) Without pre-existing immunity, the window for proliferation and differential transmission of resistant parasites is extended. White and black circles represent sensitive and resistant parasites, respectively.
Figure 5
Effect of IPT on parasite diversity. Blood concentration of drug during two treatments of IPT is shown. All of the sensitive parasites (white circles) initially present in the host at the start of IPT are killed by the drug, leaving only resistant parasites (black circles). While the drug concentration is high, new infections are prevented and the resistant parasites proliferate without competition. When the drug concentration falls below the threshold for sensitive parasites, an infectious bite can produce an infection with sensitive parasites. A subsequent bloodmeal may take up gametocytes from both sensitive (white crescents) and resistant strains (black crescents), thereby allowing out-crossing between them. At the next treatment, the sensitive parasites are killed and the resistant parasites continue to proliferate and may acquire new genetic material at the next cycle. Black arrows at the top represent continual biting by infectious mosquitoes.
Similar articles
- Potential impact of intermittent preventive treatment (IPT) on spread of drug-resistant malaria.
O'Meara WP, Smith DL, McKenzie FE. O'Meara WP, et al. PLoS Med. 2006 May;3(5):e141. doi: 10.1371/journal.pmed.0030141. Epub 2006 Apr 4. PLoS Med. 2006. PMID: 16573365 Free PMC article. - The effect of intermittent preventive treatment on anti-malarial drug resistance spread in areas with population movement.
Teboh-Ewungkem MI, Mohammed-Awel J, Baliraine FN, Duke-Sylvester SM. Teboh-Ewungkem MI, et al. Malar J. 2014 Nov 15;13:428. doi: 10.1186/1475-2875-13-428. Malar J. 2014. PMID: 25398463 Free PMC article. - Review of intermittent preventive treatment for malaria in infants and children.
Munday S. Munday S. J Paediatr Child Health. 2007 Jun;43(6):424-8. doi: 10.1111/j.1440-1754.2007.01105.x. J Paediatr Child Health. 2007. PMID: 17535170 Review. - Effect of intermittent preventive treatment for malaria during infancy on serological responses to measles and other vaccines used in the Expanded Programme on Immunization: results from five randomised controlled trials.
Crawley J, Sismanidis C, Goodman T, Milligan P; WHO Advisory Committee on serological responses to vaccines used in the Expanded Programme on Immunization in infants receiving Intermittent Preventive Treatment for malaria. Crawley J, et al. Lancet. 2012 Sep 15;380(9846):1001-10. doi: 10.1016/S0140-6736(12)60775-2. Epub 2012 Jul 30. Lancet. 2012. PMID: 22850358 - Intermittent preventive treatment against malaria: an update.
Gosling RD, Cairns ME, Chico RM, Chandramohan D. Gosling RD, et al. Expert Rev Anti Infect Ther. 2010 May;8(5):589-606. doi: 10.1586/eri.10.36. Expert Rev Anti Infect Ther. 2010. PMID: 20455687 Review.
Cited by
- Perception of Malaria Chemoprevention Interventions in Infants and Children in Eight Sub-Saharan African Countries: An End User Perspective Study.
Audibert C, Tchouatieu AM. Audibert C, et al. Trop Med Infect Dis. 2021 May 11;6(2):75. doi: 10.3390/tropicalmed6020075. Trop Med Infect Dis. 2021. PMID: 34064620 Free PMC article. - Clinical development of new prophylactic antimalarial drugs after the 5th Amendment to the Declaration of Helsinki.
Dow GS, Magill AJ, Ohrt C. Dow GS, et al. Ther Clin Risk Manag. 2008 Aug;4(4):803-19. doi: 10.2147/tcrm.s1025. Ther Clin Risk Manag. 2008. PMID: 19209263 Free PMC article. - Intermittent preventive treatment in infants as a means of malaria control: a randomized, double-blind, placebo-controlled trial in northern Ghana.
Mockenhaupt FP, Reither K, Zanger P, Roepcke F, Danquah I, Saad E, Ziniel P, Dzisi SY, Frempong M, Agana-Nsiire P, Amoo-Sakyi F, Otchwemah R, Cramer JP, Anemana SD, Dietz E, Bienzle U. Mockenhaupt FP, et al. Antimicrob Agents Chemother. 2007 Sep;51(9):3273-81. doi: 10.1128/AAC.00513-07. Epub 2007 Jul 16. Antimicrob Agents Chemother. 2007. PMID: 17638703 Free PMC article. Clinical Trial. - Presumptive treatment with sulphadoxine-pyrimethamine versus weekly chloroquine for malaria prophylaxis in children with sickle cell anaemia in Uganda: a randomized controlled trial.
Nakibuuka V, Ndeezi G, Nakiboneka D, Ndugwa CM, Tumwine JK. Nakibuuka V, et al. Malar J. 2009 Oct 24;8:237. doi: 10.1186/1475-2875-8-237. Malar J. 2009. PMID: 19852829 Free PMC article. Clinical Trial. - Malaria incidence and efficacy of intermittent preventive treatment in infants (IPTi).
Kobbe R, Adjei S, Kreuzberg C, Kreuels B, Thompson B, Thompson PA, Marks F, Busch W, Tosun M, Schreiber N, Opoku E, Adjei O, Meyer CG, May J. Kobbe R, et al. Malar J. 2007 Dec 9;6:163. doi: 10.1186/1475-2875-6-163. Malar J. 2007. PMID: 18067679 Free PMC article. Clinical Trial.
References
- Verhoeff FH, Brabin BJ, Chimsuku L, Kazembe P, Russell WB, Broadhead RL. An evaluation of the effects of intermittent sulfadoxine-pyrimethamine treatment in pregnancy on parasite clearance and risk of low birthweight in rural Malawi. Ann Trop Med Parasitol. 1998;92:141–150. doi: 10.1080/00034989859979. - DOI - PubMed
- Parise ME, Ayisi JG, Nahlen BL, Schultz LJ, Roberts JM, Misore A, Muga R, Oloo AJ, Steketee RW. Efficacy of sulfadoxlne-pyrimethamine for prevention of placental malaria in an area of Kenya with a high prevalence of malaria and human immunodeficiency virus infection. Am J Trop Med Hyg. 1998;59:813–822. - PubMed
- van Eijk AM, Ayisi JG, ter Kuile FO, Otieno JA, Misore AO, Odondi JO, Rosen DH, Kager PA, Steketee RW, Nahlen BL. Effectiveness of intermittent preventive treatment with sulphadoxine-pyrimethamine for control of malaria in pregnancy in western Kenya: a hospital-based study. Trop Med Int Health. 2004;9:351–360. doi: 10.1111/j.1365-3156.2004.01196.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical