Retrograde endocannabinoid regulation of GABAergic inhibition in the rat dentate gyrus granule cell - PubMed (original) (raw)
Retrograde endocannabinoid regulation of GABAergic inhibition in the rat dentate gyrus granule cell
Masako Isokawa et al. J Physiol. 2005.
Abstract
The dentate gyrus is a key input gateway for the hippocampus, and dentate function is potently regulated by GABAergic inhibition. GABAergic inhibition is plastic and modulated by many factors. Cytoplasmic calcium ([Ca(+)](i)) is one of these factors, and its elevation inhibits GABA-mediated transmission in the hippocampus including the dentate gyrus granule cells (DGCs). We examined whether the [Ca(+)](i)-dependent decrease of GABA(A) receptor-mediated inhibitory postsynaptic current (IPSC) is explained by the retrograde suppression of GABA release caused by the depolarization-induced elevation of [Ca(+)](i) in DGCs (DSI: depolarization-induced suppression of inhibition). Repeated brief depolarizations or a single long depolarization inhibited spontaneous IPSCs with amplitudes over 25 pA for up to a minute, and reduced the amplitude of IPSCs evoked by direct stimulation in the molecular layer, suggesting that DGCs are susceptible to DSI. The magnitude of DSI correlated linearly with the duration of depolarization, and so did the increase of [Ca(+)](i). DSI was blocked by intrapipette application of BAPTA. In addition, bath application of thapsigargin and ryanodine, and intrapipette application of ryanodine and ruthenium red reduced the [Ca(+)](i) increase caused by the DSI-inducing depolarization, and substantially reduced the magnitude of DSI. Finally, the cannabinoid receptor agonists, CP55,942 and WIN55,212-2, mimicked DSI and prevented further IPSC reduction by DSI. DSI was blocked by the antagonist, SR141716A. We conclude that GABAergic inhibition in DGCs is subject to endogenous cannabinoid (eCB)-mediated retrograde regulation, and this process involves a depolarization-initiated release of Ca(+) from ryanodine-sensitive stores. Our findings suggest eCBs probably have physiological functions in the regulation of GABAergic plasticity in the dentate gyrus.
Figures
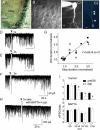
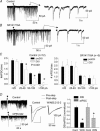
Figure 1. DGCs are capable of inducing DSI
A, dentate gyrus in hippocampal slice (calibration, 50 μm). ML, molecular layer. B, DGCs at cresta (calibration, 10 μm). C, fluorescent visualization of DGC with whole-cell recording. C1 with Lucifer yellow (calibration, 5.5 μm), and C2 with OGB488-2 (calibration, 10 μm). Arrowheads in C2 indicate regional increases in fluorescence intensity. D_–_F, DSI with depolarizing voltage steps of 1, 2, or 3 s, respectively. D, E and F share the same calibration. G, correlation between the magnitude of depolarization and that of DSI. Data were normalized. Each cell has a different symbol. H and I, blockade of DSI by BAPTA (10 m
m
). Error bars indicate standard error of the mean (
s.e.m.
), and asterisks indicate P < 0.01.
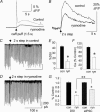
Figure 2. DSI in DGCs involves Ca2+ release from intracellular stores
A, caffeine-induced [Ca2+]i increase in control (continuous line) and in the same cell after bath application of ryanodine (20 μ
m
bath, broken line); Δ_F/F_ is a relative change in the fluoresence intensity. B, depolarization-induced [Ca2+]i increase in control (continuous line) and in the same cell after bath application of ryanodine (broken line). C, DSI recorded simultaneously with the [Ca2+]i signals shown in B. D, blockade of DSI by ryanodine accompanied by the reduction in [Ca2+]i signals shown in B. C and D share the same calibration. E, reduction in the magnitude of DSI by ryanodine (E, n = 6). F, reduction in the depolarization-induced [Ca2+]i signals by ryanodine (ryn). G, reduction in the magnitude of DSI exceeded the reduction of eIPSC amplitude in ryanodine. Data were normalized (*P < 0.0001; **P < 0.001) and error bars show
s.e.m.
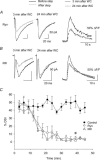
Figure 3. Intracellular application of ryanodine or ruthenium red inhibits the induction of DSI
DSI was recorded every 3 min after the establishment of whole-cell recording. DSI was apparent 3 min after the break-in with intrapipette application of ryanodine (Ryn) (A) or ruthenium red (RR) (B). However, by 24 min, DSI was reduced by these compounds. Depolarization-induced Ca2+ increase was also reduced by ryanodine (right plot in A) and ruthenium red (right plot in B). C, time-dependent decrease in the magnitude of DSI with intracellular application of ryanodine (Ryn) or ruthenium red (RR) (P < 0.01 at 40 min after the break-in). Error bars show
s.e.m.
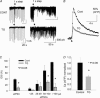
Figure 4. Depletion of Ca2+ stores downregulates the magnitude of DSI
A, thapsigargin (TG, 2 μ
m
in the bath) reduced the magnitude of sIPSC/eIPSC DSI. B, Ca2+ transients, induced by the DSI-inducing depolarizing steps, were also reduced by TG (D for group data). C, the reduction in DSI by TG was significant with sIPSCs of 50 pA in amplitude or greater. Error bars show
s.e.m.
Figure 5. DSI in DGC is CB1R dependent
A, DSI in control. B, blockade of DSI by SR141716A. Right traces in A and B are examples of sIPSCs taken from the part indicated by horizontal bars. Amplitude calibrations in A and B are all 50 pA. C, DSI affected sIPSCs with amplitudes greater than 25 pA (*P < 0.001, left graph), and was blocked by SR141716A (differences between bars not significant, 0.319 < P < 0.794; right graph). D, mimicry of DSI by the CB1R agonists, CP55,942 (left traces, sIPSCs) and WIN 55,212-2 (middle traces, eIPSCs), prevented further IPSC reduction by DSI. The reductions in the amplitudes of eIPSCs by depolarization and by WIN 55,212-2 were both significant (*P < 0.05); however, the magnitude of the reduction by depolarization and WIN 55,212-2 was not different (right graph). Error bars are
s.e.m.
Similar articles
- Endocannabinoid-mediated depolarization-induced suppression of inhibition in hilar mossy cells of the rat dentate gyrus.
Hofmann ME, Nahir B, Frazier CJ. Hofmann ME, et al. J Neurophysiol. 2006 Nov;96(5):2501-12. doi: 10.1152/jn.00310.2006. Epub 2006 Jun 28. J Neurophysiol. 2006. PMID: 16807350 - Cannabinoid receptor agonists potentiate action potential-independent release of GABA in the dentate gyrus through a CB1 receptor-independent mechanism.
Hofmann ME, Bhatia C, Frazier CJ. Hofmann ME, et al. J Physiol. 2011 Aug 1;589(Pt 15):3801-21. doi: 10.1113/jphysiol.2011.211482. Epub 2011 Jun 6. J Physiol. 2011. PMID: 21646412 Free PMC article. - Endocannabinoid- and mGluR5-dependent short-term synaptic depression in an isolated neuron/bouton preparation from the hippocampal CA1 region.
Sheinin A, Talani G, Davis MI, Lovinger DM. Sheinin A, et al. J Neurophysiol. 2008 Aug;100(2):1041-52. doi: 10.1152/jn.90226.2008. Epub 2008 May 21. J Neurophysiol. 2008. PMID: 18497350 Free PMC article. - Functional localization of cannabinoid receptors and endogenous cannabinoid production in distinct neuron populations of the hippocampus.
Hoffman AF, Riegel AC, Lupica CR. Hoffman AF, et al. Eur J Neurosci. 2003 Aug;18(3):524-34. doi: 10.1046/j.1460-9568.2003.02773.x. Eur J Neurosci. 2003. PMID: 12911748 - Dorsal Dentate Gyrus, a Key Regulator for Mood and Psychiatric Disorders.
Sun D, Mei L, Xiong WC. Sun D, et al. Biol Psychiatry. 2023 Jun 15;93(12):1071-1080. doi: 10.1016/j.biopsych.2023.01.005. Epub 2023 Jan 16. Biol Psychiatry. 2023. PMID: 36894487 Review.
Cited by
- HIV Tat Protein Selectively Impairs CB1 Receptor-Mediated Presynaptic Inhibition at Excitatory But Not Inhibitory Synapses.
Wu MM, Thayer SA. Wu MM, et al. eNeuro. 2020 Jun 19;7(3):ENEURO.0119-20.2020. doi: 10.1523/ENEURO.0119-20.2020. Print 2020 May/Jun. eNeuro. 2020. PMID: 32471847 Free PMC article. - Drug-Induced Alterations of Endocannabinoid-Mediated Plasticity in Brain Reward Regions.
Zlebnik NE, Cheer JF. Zlebnik NE, et al. J Neurosci. 2016 Oct 5;36(40):10230-10238. doi: 10.1523/JNEUROSCI.1712-16.2016. J Neurosci. 2016. PMID: 27707960 Free PMC article. Review. - Short-term plasticity regulates the excitation/inhibition ratio and the temporal window for spike integration in CA1 pyramidal cells.
Bartley AF, Dobrunz LE. Bartley AF, et al. Eur J Neurosci. 2015 May;41(11):1402-15. doi: 10.1111/ejn.12898. Epub 2015 Apr 23. Eur J Neurosci. 2015. PMID: 25903384 Free PMC article. - Anandamide potentiation of miniature spontaneous excitatory synaptic transmission is mediated via IP3 pathway.
Sang N, Zhang J, Chen C. Sang N, et al. Neurochem Int. 2010 Mar;56(4):590-6. doi: 10.1016/j.neuint.2010.01.001. Epub 2010 Jan 11. Neurochem Int. 2010. PMID: 20064571 Free PMC article. - Endocannabinoid-mediated synaptic plasticity and addiction-related behavior.
Sidhpura N, Parsons LH. Sidhpura N, et al. Neuropharmacology. 2011 Dec;61(7):1070-87. doi: 10.1016/j.neuropharm.2011.05.034. Epub 2011 Jun 12. Neuropharmacology. 2011. PMID: 21669214 Free PMC article. Review.
References
- Alger BE. Gating of GABAergic inhibition in hippocampal pyramidal cells. Activity-driven CNS changes in learning and development. Ann N Y Acad Sci. 1991;627:249–263. - PubMed
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. - PubMed
- Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992;15:303–308. - PubMed
- Carlson G, Wang Y, Alger BE. Endocannabinoids facilitate the induction of LTP in the hippocampus. Nature Neurosci. 2002;5:723–724. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources