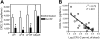G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow - PubMed (original) (raw)
G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow
Craig L Semerad et al. Blood. 2005.
Abstract
Accumulating evidence indicates that interaction of stromal cell-derived factor 1 (SDF-1/CXCL12 [CXC motif, ligand 12]) with its cognate receptor, CXCR4 (CXC motif, receptor 4), generates signals that regulate hematopoietic progenitor cell (HPC) trafficking in the bone marrow. During granulocyte colony-stimulating factor (G-CSF)-induced HPC mobilization, CXCL12 protein expression in the bone marrow decreases. Herein, we show that in a series of transgenic mice carrying targeted mutations of their G-CSF receptor and displaying markedly different G-CSF-induced HPC mobilization responses, the decrease in bone marrow CXCL12 protein expression closely correlates with the degree of HPC mobilization. G-CSF treatment induced a decrease in bone marrow CXCL12 mRNA that closely mirrored the fall in CXCL12 protein. Cell sorting experiments showed that osteoblasts and to a lesser degree endothelial cells are the major sources of CXCL12 production in the bone marrow. Interestingly, osteoblast activity, as measured by histomorphometry and osteocalcin expression, is strongly down-regulated during G-CSF treatment. However, the G-CSF receptor is not expressed on osteoblasts; accordingly, G-CSF had no direct effect on osteoblast function. Collectively, these data suggest a model in which G-CSF, through an indirect mechanism, potently inhibits osteoblast activity resulting in decreased CXCL12 expression in the bone marrow. The consequent attenuation of CXCR4 signaling ultimately leads to HPC mobilization.
Figures
Figure 1.
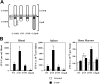
G-CSF–induced HPC mobilization in G-CSFR mutant mice. (A) Schematic of targeted G-CSFR mutations. Cytoplasmic tyrosines (Y) and the conserved box 1 and box 2 motifs are indicated. In the d715F mutant, the sole remaining tyrosine (Y704) of the G-CSFR has been mutated to phenylalanine (F). (B) Tissue distribution of HPCs following G-CSF treatment. Wild-type (WT) and G-CSFR mutant mice (n = 4, each) were treated with G-CSF (250 μg/kg/d) for 5 days and the number of CFU-Cs in blood, spleen, and bone marrow quantified 4 hours after the final dose of G-CSF. Data represent the mean ± SD. *P < .05 compared with G-CSF–treated WT mice.
Figure 2.
CXCL12α protein expression in the bone marrow following G-CSF treatment. (A) G-CSFR mutant mice (n = 7, each) were treated with G-CSF (100μg/kg/d) for 5 days and the amount of CXCL12α protein in the bone marrow extracellular fluid measured by ELISA. Data represent the mean ± SD. *P < .05 compared with untreated mice of the same genotype. (B) Plot of CXCL12α protein in the bone marrow versus the log of number of CFU-Cs in the blood on day 5 of G-CSF treatment (P < .001).
Figure 3.
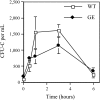
AMD3100 mobilization in GEpoR mice. Mice were treated with a single subcutaneous injection of AMD3100 (5 mg/kg). The number of CFU-Cs in the blood was measured over a 6-hour period (n = 3-4, each time point). Data represent the mean ± SD.
Figure 4.
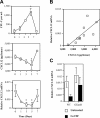
CXCL12 mRNA expression during G-CSF–induced HPC mobilization. (A) WT mice were treated with G-CSF (100 μg/kg/d) for 5 days followed by a 2-day recovery period. The number of CFU-Cs in the blood (top panel) and CXCL12 protein expression in bone marrow extracellular fluid (middle panel) were measured at the indicated time points (n = 2, each). CXCL12 mRNA expression in the bone marrow was measured by directly flushing femurs with TRIzol and performing real-time RT-PCR on the recovered RNA. Shown is the relative amount of CXCL12 mRNA compared with β-actin mRNA (bottom panel). (B) Plot of CXCL12α protein versus CXCL12 mRNA (r2 = 0.56, P < .02). (C) WT and GEpoR mice (n = 6, each) were treated with G-CSF for 5 days and CXCL12 mRNA quantified. Data represent the mean ± SD. *P < .05 compared with day 0 or untreated mice.
Figure 5.
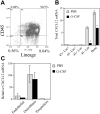
Regulation of bone marrow stromal cell activity during G-CSF–induced HPC mobilization. (A) Bone marrow cells were recovered from the femurs and tibiae of mice by flushing and treating with collagenase and then sorted into the indicated cell populations based on CD45 and lineage expression. Shown is a representative histogram. (B) To examine cells firmly adherent to the bone matrix, the flushed femurs were injected with TRIzol to obtain the “bone fraction.” Total CXCL12 mRNA in each cell population was estimated by multiplying the measured CXCL12 mRNA by the cell number in each cell fraction; the number of cells in the bone fraction was estimated based on β-actin mRNA levels. *P < .05. (C) Cells harvested from the bone fraction were sorted into the indicated cell populations (see “Materials and methods”) and CXCL12 mRNA expression relative to β-actin expression was measured. Data represent the mean ± SEM.
Figure 6.
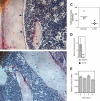
G-CSF inhibits osteoblast activity in the bone marrow. (A-D) WT mice were treated with G-CSF (125 μg/kg twice daily for 5 days) and osteoblast activity assessed. (A-B) Representative photomicrographs show endosteal osteoblasts (arrows) in untreated (A) or G-CSF–treated mice (B); original magnification × 400. (C) Quantification of osteoblast number by histomorphometry. The number of osteoblasts (N.Ob) per millimeter of bone perimeter is shown. (D) Bone marrow osteocalcin mRNA expression. Total bone marrow RNA was obtained by directly flushing femurs with TRIzol. The expression of osteocalcin mRNA relative to β-actin mRNA is shown. (E) Primary osteoblasts were cultured in the presence of 100 ng/mL G-CSF for the indicated time and CXCL12 mRNA quantified. Data represent the mean ± SEM. *P < .05. All images were obtained with a Nikon Eclipse E600 microscope using a Nikon PlanApo 20 ×/0.45 NA objective (Nikon, Melville, NY). The microscope was equipped with a Sony DXC S500 digital camera (Sony Electronics, Park Ridge, NJ), and images were captured using Kodak Imaging for Windows software (Eastman Software, Billerica, MA).
Figure 7.
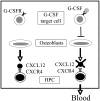
Model of G-CSF–induced HPC mobilization. Osteoblasts constitutively produce large amounts of CXCL12, providing an important retention signal for HPCs in the bone marrow. G-CSF initiates the mobilization cascade by stimulating a population of G-CSFR+ cells in the bone marrow. These cells, in turn, negatively regulate osteoblast number and activity, resulting in decreased CXCL12 expression in the bone marrow. The consequent decrease in CXCR4 signaling in HPCs leads to their migration from the bone marrow to blood.
Similar articles
- G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4.
Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T. Petit I, et al. Nat Immunol. 2002 Jul;3(7):687-94. doi: 10.1038/ni813. Epub 2002 Jun 17. Nat Immunol. 2002. PMID: 12068293 - Characterization of hematopoietic progenitor mobilization in protease-deficient mice.
Levesque JP, Liu F, Simmons PJ, Betsuyaku T, Senior RM, Pham C, Link DC. Levesque JP, et al. Blood. 2004 Jul 1;104(1):65-72. doi: 10.1182/blood-2003-05-1589. Epub 2004 Mar 9. Blood. 2004. PMID: 15010367 - Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization.
Christopher MJ, Liu F, Hilton MJ, Long F, Link DC. Christopher MJ, et al. Blood. 2009 Aug 13;114(7):1331-9. doi: 10.1182/blood-2008-10-184754. Epub 2009 Jan 13. Blood. 2009. PMID: 19141863 Free PMC article. - [The role of the SDF-1-CXCR4 axis in hematopoiesis and the mobilization of hematopoietic stem cells to peripheral blood].
Gieryng A, Bogunia-Kubik K. Gieryng A, et al. Postepy Hig Med Dosw (Online). 2007 Jun 13;61:369-83. Postepy Hig Med Dosw (Online). 2007. PMID: 17572657 Review. Polish.
Cited by
- The haematopoietic stem cell niche: new insights into the mechanisms regulating haematopoietic stem cell behaviour.
Lilly AJ, Johnson WE, Bunce CM. Lilly AJ, et al. Stem Cells Int. 2011;2011:274564. doi: 10.4061/2011/274564. Epub 2011 Oct 30. Stem Cells Int. 2011. PMID: 22135682 Free PMC article. - HIF-1α is required for hematopoietic stem cell mobilization and 4-prolyl hydroxylase inhibitors enhance mobilization by stabilizing HIF-1α.
Forristal CE, Nowlan B, Jacobsen RN, Barbier V, Walkinshaw G, Walkley CR, Winkler IG, Levesque JP. Forristal CE, et al. Leukemia. 2015 Jun;29(6):1366-78. doi: 10.1038/leu.2015.8. Epub 2015 Jan 12. Leukemia. 2015. PMID: 25578474 Free PMC article. - A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia.
Uy GL, Rettig MP, Motabi IH, McFarland K, Trinkaus KM, Hladnik LM, Kulkarni S, Abboud CN, Cashen AF, Stockerl-Goldstein KE, Vij R, Westervelt P, DiPersio JF. Uy GL, et al. Blood. 2012 Apr 26;119(17):3917-24. doi: 10.1182/blood-2011-10-383406. Epub 2012 Feb 2. Blood. 2012. PMID: 22308295 Free PMC article. Clinical Trial. - Deregulation of the CXCL12/CXCR4 axis in methotrexate chemotherapy-induced damage and recovery of the bone marrow microenvironment.
Georgiou KR, Scherer MA, King TJ, Foster BK, Xian CJ. Georgiou KR, et al. Int J Exp Pathol. 2012 Apr;93(2):104-14. doi: 10.1111/j.1365-2613.2011.00800.x. Epub 2012 Jan 5. Int J Exp Pathol. 2012. PMID: 22220905 Free PMC article. - Severe congenital neutropenia resulting from G6PC3 deficiency with increased neutrophil CXCR4 expression and myelokathexis.
McDermott DH, De Ravin SS, Jun HS, Liu Q, Priel DA, Noel P, Takemoto CM, Ojode T, Paul SM, Dunsmore KP, Hilligoss D, Marquesen M, Ulrick J, Kuhns DB, Chou JY, Malech HL, Murphy PM. McDermott DH, et al. Blood. 2010 Oct 14;116(15):2793-802. doi: 10.1182/blood-2010-01-265942. Epub 2010 Jul 8. Blood. 2010. PMID: 20616219 Free PMC article.
References
- Thomas J, Liu F, Link DC. Mechanisms of mobilization of hematopoietic progenitors with granulocyte colony-stimulating factor. Curr Opin Hematol. 2002;9: 183-189. - PubMed
- To LB, Haylock DN, Simmons PJ, Juttner CA. The biology and clinical uses of blood stem cells. Blood. 1997;89: 2233-2258. - PubMed
- Pelus LM, Horowitz D, Cooper SC, King AG. Peripheral blood stem cell mobilization. A role for CXC chemokines. Crit Rev Oncol Hematol. 2002;43: 257-275. - PubMed
- Liu F, Poursine-Laurent J, Link DC. Expression of the G-CSF receptor on hematopoietic progenitor cells is not required for their mobilization by GCSF. Blood. 2000;95: 3025-3031. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases