TcpF is a soluble colonization factor and protective antigen secreted by El Tor and classical O1 and O139 Vibrio cholerae serogroups - PubMed (original) (raw)
TcpF is a soluble colonization factor and protective antigen secreted by El Tor and classical O1 and O139 Vibrio cholerae serogroups
Thomas J Kirn et al. Infect Immun. 2005 Aug.
Abstract
Vibrio cholerae causes diarrhea by colonizing the human small bowel and intoxicating epithelial cells. Colonization is a required step in pathogenesis, and strains defective for colonization are significantly attenuated. The best-characterized V. cholerae colonization factor is the toxin-coregulated pilus (TCP). It has been demonstrated that TCP is required for V. cholerae colonization in both humans and mice. TCP enhances bacterial interactions that allow microcolony formation and thereby promotes survival in the intestine. We have recently discovered that the TCP biogenesis apparatus also serves as a secretion system, mediating the terminal step in the extracellular secretion pathway of TcpF. TcpF was identified in classical isolates of V. cholerae O1 as a soluble factor essential for colonization in the infant mouse cholera model. In the present study, we expanded our analysis of TcpF to include the O1 El Tor and O139 serogroups and investigated how TCP and TcpF act together to mediate colonization. Additionally, we demonstrated that antibodies generated against TcpF are protective against experimental V. cholerae infection in the infant mouse cholera model. This observation, coupled with the fact that TcpF is a potent mediator of colonization, suggests that TcpF should be considered as a component of a polyvalent cholera vaccine formulation.
Figures
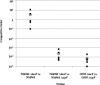
FIG. 1.
Secretion of TcpF from V. cholerae. (A) Determination of TcpF and TcpA levels in whole-cell extracts (wc) and culture supernatants (cs) from V. cholerae O395 (wt) and O395 Δ_tcpF_ (Δ_tcpF_). The numbers below the lanes indicate the total amounts of protein loaded as determined by the bicinchoninic acid protein assay (Pierce). (B) Immunoblot analysis of culture supernatants from strains representing each epidemic serogroup (O1 and O139), the two biotypes of O1, and the two serotypes of classical O1 V. cholerae. The primary antiserum was generated using a purified TcpF-GST fusion protein.
FIG. 2.
In vivo competition of wild-type and tcpF mutants in the infant mouse cholera model. Δ_tcpF_ mutations were generated in O1 El Tor (N16961) and classical (O395) strains and used in competition assays with the corresponding wild-type parental strains. The competitive index for each mouse (▪) and the average for each group (○) are shown.
FIG. 3.
TcpF secretion and TCP biogenesis in the E158L tcpA point mutant. (A) Culture supernatants isolated from wild-type, Δ_tcpF_, and E158L strains were analyzed for TcpF secretion by SDS-PAGE and immunoblotting with anti-TcpF antiserum. (B) Transmission electron micrograph showing TCP elaborated by wild-type V. cholerae (wt) and the nonfunctional, morphologically aberrant pili elaborated by the E158L mutant. (C) Relevant in vitro and in vivo features of wild-type, Δ_tcpA_, E158L, and Δ_tcpF_ strains. The presence of stable pilin, pilus features, autoagglutination (AA), serum resistance, CTX phage transduction efficiency (CTXφ Tx), and competitive indices (CI) were determined as previously described (24).
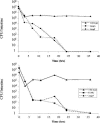
FIG. 4.
Kinetics of bacterial clearance from the infant mouse intestine. (Top panel) Wild-type, Δ_tcpA_, or Δ_tcpF_ bacteria were individually inoculated into infant mice, and the number of bacteria colonizing the small bowel was determined at various times. Each point represents the average of two mice. (Bottom panel) Wild-type, E158L, and Δ_tcpF_ samples were each mixed with an equal volume of wild-type bacteria (which were Δ_lacZ_ mutants) prior to inoculation, and the numbers of LacZ+ colonies remaining in the intestine at various times were determined. Each point represents the average of two mice.
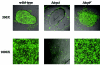
FIG. 5.
Wild-type in vitro microcolony formation compared to microcolony formation by the Δ_tcpA_ and Δ_tcpF_ mutants. Wild-type V. cholerae was grown under TCP-inducing conditions along with wild-type, Δ_tcpA_, and Δ_tcpF V. cholerae_ strains bearing a plasmid encoding GFP. The images of the mixed microcolonies include fluorescein isothiocyanate channel signals (green) overlaying differential interference contrast signals (grey).
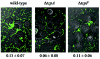
FIG. 6.
Attachment of V. cholerae mutants to HT-29 epithelial cells. Wild-type, Δ_tcpA_, and Δ_tcpF_ strains bearing a plasmid encoding GFP were inoculated into tissue culture wells containing a confluent layer of HT-29 human colonic epithelial cells. After growth for 4 h, representative photographs were taken, and the number of attached bacteria was determined by appropriately diluting and plating bacteria. The numbers below the micrographs are the ratios of the number of bacteria attached to epithelial cells to the input numbers (means ± standard deviations).
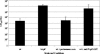
FIG. 7.
Protection of infant mice from experimental cholera infection by passive immunization with anti-TcpF antiserum. Dilutions of overnight cultures of wild-type V. cholerae (wt) were mixed with either preimmune or hyperimmune sera from rabbits immunized with purified TcpF-GST. The bars indicate the LD50 at 48 h.
FIG. 8.
Proposed model for V. cholerae intestinal colonization. We propose the following three-step model for colonization: (i) initial attachment (step 1), (ii) microcolony formation (step 2), and (iii) macrocolony formation and maintenance (step 3). Factors that may be involved in each of these steps are listed. Relevant micrographs show GFP-expressing V. cholerae (green) colonizing HT-29 cells (grey) in vitro after 1 and 4 h of incubation; for step 1 the magnification is ×400, and for step 2 the magnification is ×1,000. The micrograph for step 3 shows an infant mouse intestine (blue) colonized by GFP-expressing V. cholerae (green) 16 h after oral inoculation (magnification, ×200).
Similar articles
- Genetic mapping of secretion and functional determinants of the Vibrio cholerae TcpF colonization factor.
Krebs SJ, Kirn TJ, Taylor RK. Krebs SJ, et al. J Bacteriol. 2009 Jun;191(11):3665-76. doi: 10.1128/JB.01724-08. Epub 2009 Mar 20. J Bacteriol. 2009. PMID: 19304855 Free PMC article. - Crystal structure of the Vibrio cholerae colonization factor TcpF and identification of a functional immunogenic site.
Megli CJ, Yuen AS, Kolappan S, Richardson MR, Dharmasena MN, Krebs SJ, Taylor RK, Craig L. Megli CJ, et al. J Mol Biol. 2011 Jun 3;409(2):146-58. doi: 10.1016/j.jmb.2011.03.027. Epub 2011 Apr 1. J Mol Biol. 2011. PMID: 21440558 Free PMC article. - The major subunit of the toxin-coregulated pilus TcpA induces mucosal and systemic immunoglobulin A immune responses in patients with cholera caused by Vibrio cholerae O1 and O139.
Asaduzzaman M, Ryan ET, John M, Hang L, Khan AI, Faruque AS, Taylor RK, Calderwood SB, Qadri F. Asaduzzaman M, et al. Infect Immun. 2004 Aug;72(8):4448-54. doi: 10.1128/IAI.72.8.4448-4454.2004. Infect Immun. 2004. PMID: 15271902 Free PMC article. - Pathogenic and vaccine significance of toxin-coregulated pili of Vibrio cholerae E1 Tor.
Attridge SR, Voss E, Manning PA. Attridge SR, et al. J Biotechnol. 1999 Aug 20;73(2-3):109-17. doi: 10.1016/s0168-1656(99)00114-5. J Biotechnol. 1999. PMID: 10486921 Review. - Epidemiology & molecular biology of Vibrio cholerae O139 Bengal.
Albert MJ. Albert MJ. Indian J Med Res. 1996 Jul;104:14-27. Indian J Med Res. 1996. PMID: 8783504 Review.
Cited by
- Vibrio cholerae: lessons for mucosal vaccine design.
Bishop AL, Camilli A. Bishop AL, et al. Expert Rev Vaccines. 2011 Jan;10(1):79-94. doi: 10.1586/erv.10.150. Expert Rev Vaccines. 2011. PMID: 21162623 Free PMC article. Review. - Genetic mapping of secretion and functional determinants of the Vibrio cholerae TcpF colonization factor.
Krebs SJ, Kirn TJ, Taylor RK. Krebs SJ, et al. J Bacteriol. 2009 Jun;191(11):3665-76. doi: 10.1128/JB.01724-08. Epub 2009 Mar 20. J Bacteriol. 2009. PMID: 19304855 Free PMC article. - Lipidation of an FlrC-dependent protein is required for enhanced intestinal colonization by Vibrio cholerae.
Morris DC, Peng F, Barker JR, Klose KE. Morris DC, et al. J Bacteriol. 2008 Jan;190(1):231-9. doi: 10.1128/JB.00924-07. Epub 2007 Nov 2. J Bacteriol. 2008. PMID: 17981980 Free PMC article. - Nutrient-dependent, rapid transition of Vibrio cholerae to coccoid morphology and expression of the toxin co-regulated pilus in this form.
Krebs SJ, Taylor RK. Krebs SJ, et al. Microbiology (Reading). 2011 Oct;157(Pt 10):2942-2953. doi: 10.1099/mic.0.048561-0. Epub 2011 Jul 21. Microbiology (Reading). 2011. PMID: 21778208 Free PMC article. - Protection and attachment of Vibrio cholerae mediated by the toxin-coregulated pilus in the infant mouse model.
Krebs SJ, Taylor RK. Krebs SJ, et al. J Bacteriol. 2011 Oct;193(19):5260-70. doi: 10.1128/JB.00378-11. Epub 2011 Jul 29. J Bacteriol. 2011. PMID: 21804008 Free PMC article.
References
- Braunwald, E. 2001. Harrison's principles of internal medicine, 15th ed. McGraw-Hill, New York, N.Y.
- Cash, R. A., S. I. Music, J. P. Libonati, M. J. Snyder, R. P. Wenzel, and R. B. Hornick. 1974. Response of man to infection with Vibrio cholerae. I. Clinical, serologic, and bacteriologic responses to a known inoculum. J. Infect. Dis. 129:45-52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007519/AI/NIAID NIH HHS/United States
- AI25096/AI/NIAID NIH HHS/United States
- R01 AI025096/AI/NIAID NIH HHS/United States
- AI007519/AI/NIAID NIH HHS/United States
- R37 AI025096/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources