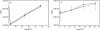Specificity of Legionella pneumophila and Coxiella burnetii vacuoles and versatility of Legionella pneumophila revealed by coinfection - PubMed (original) (raw)
Specificity of Legionella pneumophila and Coxiella burnetii vacuoles and versatility of Legionella pneumophila revealed by coinfection
John-Demian Sauer et al. Infect Immun. 2005 Aug.
Abstract
Legionella pneumophila and Coxiella burnetii are phylogenetically related intracellular bacteria that cause aerosol-transmitted lung infections. In host cells both pathogens proliferate in vacuoles whose biogenesis displays some common features. To test the functional similarity of their respective intracellular niches, African green monkey kidney epithelial (Vero) cells, A/J mouse bone marrow-derived macrophages, human macrophages, and human dendritic cells (DC) containing mature C. burnetii replication vacuoles were superinfected with L. pneumophila, and then the acidity, lysosome-associated membrane protein (LAMP) content, and cohabitation of mature replication vacuoles was assessed. In all cell types, wild-type L. pneumophila occupied distinct vacuoles in close association with acidic, LAMP-positive C. burnetii replication vacuoles. In murine macrophages, but not primate macrophages, DC, or epithelial cells, L. pneumophila replication vacuoles were acidic and LAMP positive. Unlike wild-type L. pneumophila, type IV secretion-deficient dotA mutants trafficked to lysosome-like C. burnetii vacuoles in Vero cells where they survived but failed to replicate. In primate macrophages, DC, or epithelial cells, growth of L. pneumophila was as robust in superinfected cell cultures as in those singly infected. Thus, despite their noted similarities, L. pneumophila and C. burnetii are exquisitely adapted for replication in unique replication vacuoles, and factors that maintain the C. burnetii replication vacuole do not alter biogenesis of an adjacent L. pneumophila replication vacuole. Moreover, L. pneumophila can replicate efficiently in either lysosomal vacuoles of A/J mouse cells or in nonlysosomal vacuoles of primate cells.
Figures
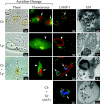
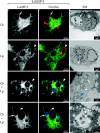
FIG. 1.
Replication vacuole formation in Vero cells infected with C. burnetii and/or L. pneumophila. Vero cells infected with C. burnetii phase II (Cb; arrows) for 48 h were superinfected with wild-type L. pneumophila (Lp; arrowheads) or the isogenic dotA strain (Lp dotA; arrowheads) for 24 h. Vero cells singly infected with C. burnetii for 72 h or L. pneumophila for 24 h were used as controls. Live cells were stained with the acidotropic base acridine orange, and the same field was viewed by phase-contrast and fluorescence microscopy. Fixed cells were examined for localization of the lysosomal glycoprotein LAMP-1 by indirect immunofluorescence. Representative electron micrographs of infected cells are also depicted. In both singly and superinfected cells, acridine orange was sequestered by replication vacuoles harboring C. burnetii but not L. pneumophila. In singly infected cells, LAMP-1 (green) localized to the membrane of distinct replication vacuoles harboring C. burnetii (red) but not L. pneumophila (red). In superinfected cells, LAMP-1 (green) also localized to the C. burnetii (blue) replication vacuole membrane but not to the L. pneumophila (red) replication vacuole membrane. C. burnetii and L. pneumophila were never observed in the same replication vacuole. In _C. burnetii_-infected cells superinfected with L. pneumphila dotA type IV secretion mutants, nonreplicating L. pneumophila (red) localized to the LAMP-1-positive (green) vacuole containing replicating C. burnetii (blue). Vacuoles harboring both L. pneumophila dotA and C. burnetii were observed in 60.7% ± 5.0% of cells infected with both pathogens. In electron micrographs, L. pneumophila are distinguished from C. burnetii by their larger size (>1 μm in length), uniform rod morphology, electron-translucent hydroxybutyrate granules, and lack of filamentous chromatin. Percent colocalization of dotA mutants is expressed as the mean ± the standard deviation of three independent experiments in which at least 50 superinfected cells were evaluated. Images are representative of three independent experiments in which at least 100 singly infected or superinfected cells were examined.
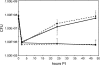
FIG. 2.
Growth of L. pneumophila in _C. burnetii_-infected Vero cells. Vero cells infected with C. burnetii (phase II) for 48 h were superinfected with wild-type L. pneumophila or the isogenic dotA mutant. Vero cells were singly infected with L. pneumophila as a control. Intracellular L. pneumophila were released from host cells at 2, 24, and 48 h p.i., and CFU assays performed. The 0 h time point represents the titer of the starting inoculum prior to washing extracellular organisms from the monolayer at 2 h p.i. Wild-type L. pneumophila CFU increased 53- and 86-fold between 2 and 48 h p.i. in singly infected (solid line with squares) and superinfected cells (dotted line with squares), respectively. No replication of the dotA mutant was observed in singly infected (dotted line with circles) or superinfected (solid line with circles) cells. The results are expressed as the mean from three independent experiments with error bars representing the standard deviation.
FIG. 3.
Replication vacuole formation in murine BMDM infected with C. burnetii and/or L. pneumophila. BMDM from A/J mice were infected with phase I C. burnetii (Cb I; arrows) for 48 h and then superinfected with L. pneumophila (Lp; arrowheads) for 18 h. BMDM singly infected with phase I or phase II C. burnetii (Cb II; arrows) for 66 h or L. pneumophila for 18 h were also evaluated. Live cells were stained with the acidotropic base acridine orange, and the same field was viewed by phase-contrast and fluorescence microscopy. Fixed cells were examined for localization of the lysosomal glycoprotein LAMP-2 by indirect immunofluorescence. In singly infected cells, acridine orange was sequestered by replication vacuoles harboring both phase I C. burnetii and L. pneumophila. In singly infected cells, LAMP-2 (green) localized to the vacuole membrane surrounding both phase II (red) and phase I C. burnetii (red), as well as L. pneumophila (red). In phase II _C. burnetii_-infected cells, single organisms were observed scattered throughout the cytoplasm tightly bounded by a LAMP-2-positive membrane. In C. burnetii phase I- or _L. pneumophila_-infected cells, replicating organisms were observed in multiple large replication vacuoles. In superinfected cells, LAMP-2 (green) also localized to vacuoles harboring phase I C. burnetii (blue) and L. pneumophila (red). In no instance were phase I C. burnetii and L. pneumophila observed in the same vacuole. Images are representative of three independent experiments where at least 100 singly infected or superinfected cells were examined.
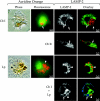
FIG. 4.
Replication vacuole formation in human primary macrophages infected with C. burnetii and/or L. pneumophila. Cells were infected with phase II C. burnetii (Cb; arrows) for 36 h and then superinfected with L. pneumophila (Lp; arrowheads) for 12 h. Cells singly infected with C. burnetii for 48 h or L. pneumophila for 12 h were used as controls. Fixed cells were examined for localization of the lysosomal glycoprotein LAMP-1 by indirect immunofluorescence. In singly infected cells, LAMP-1 (green) localized to the membrane of distinct replication vacuoles harboring C. burnetii (red) but not L. pneumophila (red). In superinfected cells, LAMP-1 (green) also localized to the C. burnetii (blue) replication vacuole membrane but not to the L. pneumophila (red) replication vacuole membrane. In no instance were C. burnetii and L. pneumophila observed in the same replication vacuole. Images are representative of three independent experiments in which at least 100 singly infected or superinfected cells were examined.
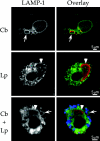
FIG.5.
Replication vacuole formation in human primary DC infected with C. burnetii and/or L. pneumophila. Cells were infected with phase II C. burnetii (Cb; arrows) for 36 h and then superinfected with L. pneumophila (Lp; arrowheads) for 12 h. Cells singly infected with C. burnetii for 48 h or L. pneumophila for 12 h were used as controls. Fixed cells were examined for localization of the lysosomal glycoprotein LAMP-1 by indirect immunofluorescence. Representative electron micrographs of infected cells are also depicted. In singly infected cells, LAMP-1 (green) localized to the membrane of distinct replication vacuoles harboring C. burnetii (red) but not L. pneumophila (red). In superinfected cells, LAMP-1 (green) also localized to distinct vacuoles harboring replicating C. burnetii (blue) but not replicating L. pneumophila. On rare occasions one or two L. pneumophila organisms were observed in C. burnetii replication vacuoles in superinfected cells (lower dual-infection panels). L. pneumophila are distinguished from C. burnetii in electron micrographs by their larger size (>1 μm in length), uniform rod morphology, electron-translucent poly-3-hydroxybutyrate granules, and lack of filamentous chromatin. Images are representative of three independent experiments where at least 100 singly infected or superinfected cells were examined.
FIG. 6.
Growth of L. pneumophila in _C. burnetii_-infected primary monocyte-derived macrophages and DC. Intracellular L. pneumophila were released from host cells and CFU were quantified to assay replication in singly infected macrophages and DC and in cells infected with C. burnetii (phase II) for 36 h. The 0-h time point represents the titer of the starting inoculum prior to washing extracellular organisms from the monolayer at 2 h p.i. (This wash step was not conducted with nonadherent DC cultures.) (A) L. pneumophila CFU increased 188 and 165-fold between 2 and 48 h p.i. in singly infected (solid line with squares) and superinfected macrophages (dotted line with squares), respectively. (B) L. pneumophila CFU increased 27- and 16-fold between 2 and 72 h p.i. in singly infected (solid line with triangles) and superinfected DC (dotted line with triangles), respectively. The results are expressed as the means from three independent experiments, with error bars representing the standard deviations.
Similar articles
- The role of Rab GTPases in the transport of vacuoles containing Legionella pneumophila and Coxiella burnetii.
Hardiman CA, McDonough JA, Newton HJ, Roy CR. Hardiman CA, et al. Biochem Soc Trans. 2012 Dec 1;40(6):1353-9. doi: 10.1042/BST20120167. Biochem Soc Trans. 2012. PMID: 23176480 Review. - Evolution and function of bacterial RCC1 repeat effectors.
Swart AL, Gomez-Valero L, Buchrieser C, Hilbi H. Swart AL, et al. Cell Microbiol. 2020 Oct;22(10):e13246. doi: 10.1111/cmi.13246. Epub 2020 Aug 26. Cell Microbiol. 2020. PMID: 32720355 Review. - The Polar Legionella Icm/Dot T4SS Establishes Distinct Contact Sites with the Pathogen Vacuole Membrane.
Böck D, Hüsler D, Steiner B, Medeiros JM, Welin A, Radomska KA, Hardt WD, Pilhofer M, Hilbi H. Böck D, et al. mBio. 2021 Oct 26;12(5):e0218021. doi: 10.1128/mBio.02180-21. Epub 2021 Oct 12. mBio. 2021. PMID: 34634944 Free PMC article. - Coxiella burnetii express type IV secretion system proteins that function similarly to components of the Legionella pneumophila Dot/Icm system.
Zamboni DS, McGrath S, Rabinovitch M, Roy CR. Zamboni DS, et al. Mol Microbiol. 2003 Aug;49(4):965-76. doi: 10.1046/j.1365-2958.2003.03626.x. Mol Microbiol. 2003. PMID: 12890021 - Dependency of Coxiella burnetii Type 4B Secretion on the Chaperone IcmS.
Larson CL, Beare PA, Heinzen RA. Larson CL, et al. J Bacteriol. 2019 Nov 5;201(23):e00431-19. doi: 10.1128/JB.00431-19. Print 2019 Dec 1. J Bacteriol. 2019. PMID: 31501284 Free PMC article.
Cited by
- Molecular pathogenesis of the obligate intracellular bacterium Coxiella burnetii.
van Schaik EJ, Chen C, Mertens K, Weber MM, Samuel JE. van Schaik EJ, et al. Nat Rev Microbiol. 2013 Aug;11(8):561-73. doi: 10.1038/nrmicro3049. Epub 2013 Jun 24. Nat Rev Microbiol. 2013. PMID: 23797173 Free PMC article. Review. - Host-bacteria interactions: ecological and evolutionary insights from ancient, professional endosymbionts.
Bontemps Z, Paranjape K, Guy L. Bontemps Z, et al. FEMS Microbiol Rev. 2024 Jun 20;48(4):fuae021. doi: 10.1093/femsre/fuae021. FEMS Microbiol Rev. 2024. PMID: 39081075 Free PMC article. Review. - Host and Bacterial Factors Control Susceptibility of Drosophila melanogaster to Coxiella burnetii Infection.
Bastos RG, Howard ZP, Hiroyasu A, Goodman AG. Bastos RG, et al. Infect Immun. 2017 Jun 20;85(7):e00218-17. doi: 10.1128/IAI.00218-17. Print 2017 Jul. Infect Immun. 2017. PMID: 28438980 Free PMC article. - The many forms of a pleomorphic bacterial pathogen-the developmental network of Legionella pneumophila.
Robertson P, Abdelhady H, Garduño RA. Robertson P, et al. Front Microbiol. 2014 Dec 22;5:670. doi: 10.3389/fmicb.2014.00670. eCollection 2014. Front Microbiol. 2014. PMID: 25566200 Free PMC article. Review. - Trypanosoma cruzi Differentiates and Multiplies within Chimeric Parasitophorous Vacuoles in Macrophages Coinfected with Leishmania amazonensis.
Pessoa CC, Ferreira ÉR, Bayer-Santos E, Rabinovitch M, Mortara RA, Real F. Pessoa CC, et al. Infect Immun. 2016 Apr 22;84(5):1603-1614. doi: 10.1128/IAI.01470-15. Print 2016 May. Infect Immun. 2016. PMID: 26975994 Free PMC article.
References
- Andreoli, W. K., and R. A. Mortara. 2003. Acidification modulates the traffic of Trypanosoma cruzi trypomastigotes in Vero cells harbouring Coxiella burnetii vacuoles. Int. J. Parasitol. 33:185-197. - PubMed
- Bachman, M. A., and M. S. Swanson. 2001. RpoS co-operates with other factors to induce Legionella pneumophila virulence in the stationary phase. Mol. Microbiol. 40:1201-1214. - PubMed
- Berger, K. H., and R. R. Isberg. 1993. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol. 7:7-19. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical