Essential role for survivin in early brain development - PubMed (original) (raw)
Essential role for survivin in early brain development
Yuying Jiang et al. J Neurosci. 2005.
Abstract
Apoptosis is an essential process during normal neuronal development. Approximately one-half of the neurons produced during neurogenesis die before completion of CNS maturation. To characterize the role of the inhibitor of apoptosis gene, survivin, during neurogenesis, we used the Cre-loxP-system to generate mice lacking survivin in neuronal precursor cells. Conditional deletion of survivin starting at embryonic day 10.5 leads to massive apoptosis of neuronal precursor cells in the CNS. Conditional mutants were born at the expected Mendelian ratios; however, these died shortly after birth from respiratory insufficiency, without primary cardiopulmonary pathology. Newborn conditional mutants showed a marked reduction in the size of the brain associated with severe, mutifocal apoptosis in the cerebrum, cerebellum, brainstem, spinal cord, and retina. Caspase-3 and caspase-9 activities in the mutant brains were significantly elevated, whereas bax expression was unchanged from controls. These results show that survivin is critically required for the survival of developing CNS neurons, and may impact on our understanding of neural repair, neural development, and neurodegenerative diseases. Our study is the first to solidify a role for survivin as an antiapoptotic protein during normal neuronal development in vivo.
Figures
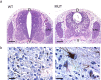
Figure 1.
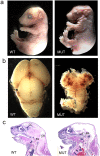
Brain malformation in nestin-cre;survivinlox/lox mice. a, Whole mounts of E17.5 mice showing the abnormal head morphology of a mutant (MUT) compared with a wild-type (WT) littermate. b, Dissected brain from mutant and wild-type embryos at E17.5. c, Sagittal H&E-stained sections of mice at P0 showing the brain structures of survivin mutant and wild-type animals. The cerebral cortex (CC) and cerebellum (CB) were absent in the mutant brains. The brainstem (BS) was relatively preserved but smaller in the mutant than in the wild-type animals.
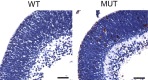
Figure 2.
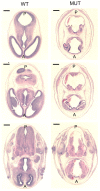
H&E-stained transverse sections at E13.5 showing multifocal destruction of brain architecture within the developing brain of the mutant embryos. Scale bars, 50 μm. WT, Wild type; MUT, mutant; A, anterior; P, posterior.
Figure 3.
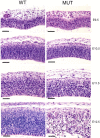
H&E-stained sections from pial (top) to ventricular (bottom) surface at E9.5-E12.5 showing progressive destruction of cortical architecture within the developing cerebral cortex of the mutant embryos. Scale bars, 500 μm. WT, Wild type; MUT, mutant.
Figure 4.
Top, TUNEL assays showing progressive increases in apoptotic cell death within the cerebral cortex of the mutant embryos, starting at E10.5. Scale bars, 500 μm. Bottom, A histogram depicts the average number of TUNEL-positive cells per area for the wild-type (WT) and mutant (MUT) animals. *Statistically significant difference (p = 0.01). Error bars indicate SD.
Figure 5.
Top, PCNA staining showing small differences in the number of proliferating cells within the cerebral cortex between mutant and wild-type animals. Scale bars, 500 μm. Bottom, A histogram depicts the average number of PCNA-positive cells per area for the wild-type (WT) and mutant (MUT) animals. No statistical differences were observed. Error bars indicate SD.
Figure 6.
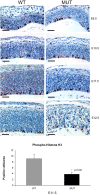
Top, PHH3 expression showing a moderate decrease in the number of cells in G2/M in the cerebral cortex of the mutant embryos. Scale bars, 500 μm. Bottom, A histogram depicts the average number of PHH3-positive cells per area for the wild-type (WT) and mutant (MUT) animals. *Statistically significant difference (p = 0.02). Error bars indicate SD.
Figure 7.
Activated caspase expression in the developing cortex of nestin-cre;survivinlox/lox embryos. Top, Sections from the cerebral cortex from E9.5 through E12.5 were stained with activated caspase-3 antibodies. A progressive increase in expression of activated caspase-3 is seen, starting at E10.5. Scale bars, 50 μm. Bottom, A histogram depicts the average number of caspase-3-positive cells per area for the wild-type (WT) and mutant (MUT) animals. *Statistically significant difference (p < 0.001). Error bars indicate SD.
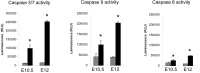
Figure 8.
Functional caspase activity in the developing cortex of nestin-cre;survivinlox/lox embryos. Developing cortical tissues were evaluated for functional caspase-3/7, -8, and -9 activities at E10.5 and E12 using a luciferase-based chemical assay system (Caspase-Glo; Promega). Relative luciferase units (RLU) are proportional to functional caspase activity, as indicated by the manufacturer. Gray columns represent the samples from wild-type animals, and black columns represent the samples from the mutants. Three animals in each group were used (n = 3). *Statistical significance (p < 0.001 for all). Error bars indicate SD.
Figure 9.
Effects of a lack of survivin within the developing spinal cord. a, Sections of the developing spinal cord from E12 embryos were stained with H&E. Neuronal disorganization and cell death is observed. DRG, Dorsal root ganglia; D, dorsal; V, ventral. Scale bars, 100 μm. b, Sections of spinal cords from mutant (MUT) mice and wild-type (WT) littermates at P0 were stained with GFAP. Giant cells observed in the mutant animals expressed GFAP, suggesting they are of an astrocytic lineage. Scale bars, 25 μm.
Figure 10.
Retinal defects in nestin-cre;survivinlox/lox mice. TUNEL assays show increased apoptosis in the retina at E17.5 in the nestin-cre;survivinlox/lox embryos. Apoptotic nuclei appear dark red. Scale bars, 50 μm. WT, Wild type; MUT, mutant.
Similar articles
- IGF-IR-dependent expression of Survivin is required for T-antigen-mediated protection from apoptosis and proliferation of neural progenitors.
Gualco E, Urbanska K, Perez-Liz G, Sweet T, Peruzzi F, Reiss K, Del Valle L. Gualco E, et al. Cell Death Differ. 2010 Mar;17(3):439-51. doi: 10.1038/cdd.2009.146. Epub 2009 Oct 16. Cell Death Differ. 2010. PMID: 19834489 Free PMC article. - Impaired neurogenesis, learning and memory and low seizure threshold associated with loss of neural precursor cell survivin.
Coremans V, Ahmed T, Balschun D, D'Hooge R, DeVriese A, Cremer J, Antonucci F, Moons M, Baekelandt V, Reumers V, Cremer H, Eisch A, Lagace D, Janssens T, Bozzi Y, Caleo M, Conway EM. Coremans V, et al. BMC Neurosci. 2010 Jan 5;11:2. doi: 10.1186/1471-2202-11-2. BMC Neurosci. 2010. PMID: 20051123 Free PMC article. - Downregulation of survivin regulates adult hippocampal neurogenesis and apoptosis, and inhibits spatial learning and memory following traumatic brain injury.
Zhang Z, Wang H, Jin Z, Cai X, Gao N, Cui X, Liu P, Zhang J, Yang S, Yang X. Zhang Z, et al. Neuroscience. 2015 Aug 6;300:219-28. doi: 10.1016/j.neuroscience.2015.05.025. Epub 2015 May 16. Neuroscience. 2015. PMID: 25987205 - Survivin: a protein with dual roles in mitosis and apoptosis.
Wheatley SP, McNeish IA. Wheatley SP, et al. Int Rev Cytol. 2005;247:35-88. doi: 10.1016/S0074-7696(05)47002-3. Int Rev Cytol. 2005. PMID: 16344111 Review. - [Research advances on inhibitor of apoptosis, survivin].
Yang J, Liu FX, Yan XC. Yang J, et al. Ai Zheng. 2003 Jul;22(7):771-4. Ai Zheng. 2003. PMID: 12866973 Review. Chinese.
Cited by
- Targeted disruption in mice of a neural stem cell-maintaining, KRAB-Zn finger-encoding gene that has rapidly evolved in the human lineage.
Chien HC, Wang HY, Su YN, Lai KY, Lu LC, Chen PC, Tsai SF, Wu CI, Hsieh WS, Shen CK. Chien HC, et al. PLoS One. 2012;7(10):e47481. doi: 10.1371/journal.pone.0047481. Epub 2012 Oct 10. PLoS One. 2012. PMID: 23071813 Free PMC article. - Loss of Cdk5 function in the nucleus accumbens decreases wheel running and may mediate age-related declines in voluntary physical activity.
Ruegsegger GN, Toedebusch RG, Childs TE, Grigsby KB, Booth FW. Ruegsegger GN, et al. J Physiol. 2017 Jan 1;595(1):363-384. doi: 10.1113/JP272489. Epub 2016 Sep 15. J Physiol. 2017. PMID: 27461471 Free PMC article. - Inhibitor of apoptosis proteins in eukaryotic evolution and development: a model of thematic conservation.
O'Riordan MX, Bauler LD, Scott FL, Duckett CS. O'Riordan MX, et al. Dev Cell. 2008 Oct;15(4):497-508. doi: 10.1016/j.devcel.2008.09.012. Dev Cell. 2008. PMID: 18854135 Free PMC article. Review. - CDK5RAP2 interaction with components of the Hippo signaling pathway may play a role in primary microcephaly.
Sukumaran SK, Stumpf M, Salamon S, Ahmad I, Bhattacharya K, Fischer S, Müller R, Altmüller J, Budde B, Thiele H, Tariq M, Malik NA, Nürnberg P, Baig SM, Hussain MS, Noegel AA. Sukumaran SK, et al. Mol Genet Genomics. 2017 Apr;292(2):365-383. doi: 10.1007/s00438-016-1277-x. Epub 2016 Dec 21. Mol Genet Genomics. 2017. PMID: 28004182 Free PMC article. - Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson's disease models.
Song B, Cha Y, Ko S, Jeon J, Lee N, Seo H, Park KJ, Lee IH, Lopes C, Feitosa M, Luna MJ, Jung JH, Kim J, Hwang D, Cohen BM, Teicher MH, Leblanc P, Carter BS, Kordower JH, Bolshakov VY, Kong SW, Schweitzer JS, Kim KS. Song B, et al. J Clin Invest. 2020 Feb 3;130(2):904-920. doi: 10.1172/JCI130767. J Clin Invest. 2020. PMID: 31714896 Free PMC article.
References
- Altieri DC (2003) Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene 22: 8581-8589. - PubMed
- Ambrosini G, Adida C, Altieri DC (1997) A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 3: 917-921. - PubMed
- Ambrosini G, Adida C, Sirugo G, Altieri DC (1998) Induction of apoptosis and inhibition of cell proliferation by survivin gene targeting. J Biol Chem 273: 11177-11182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials