A mechanism for Ca2+/calmodulin-dependent protein kinase II clustering at synaptic and nonsynaptic sites based on self-association - PubMed (original) (raw)
A mechanism for Ca2+/calmodulin-dependent protein kinase II clustering at synaptic and nonsynaptic sites based on self-association
Andy Hudmon et al. J Neurosci. 2005.
Abstract
The activity of Ca2+/calmodulin-dependent protein kinase II (CaMKII) plays an integral role in regulating synaptic development and plasticity. We designed a live-cell-imaging approach to monitor an activity-dependent clustering of green fluorescent protein (GFP)-CaMKII holoenzymes, termed self-association, a process that we hypothesize contributes to the translocation of CaMKII to synaptic and nonsynaptic sites in activated neurons. We show that GFP-CaMKII self-association in human embryonic kidney 293 (HEK293) cells requires a catalytic domain and multimeric structure, requires Ca2+ stimulation and a functional Ca2+/CaM-binding domain, is regulated by cellular pH and Thr286 autophosphorylation, and has variable rates of dissociation depending on Ca2+ levels. Furthermore, we show that the same rules that govern CaMKII self-association in HEK293 cells apply for extrasynaptic and postsynaptic translocation of GFP-CaMKII in hippocampal neurons. Our data support a novel mechanism for targeting CaMKII to postsynaptic sites after neuronal activation. As such, CaMKII may form a scaffold that, in combination with other synaptic proteins, recruits and localizes additional proteins to the postsynaptic density. We discuss the potential function of CaMKII self-association as a tag of synaptic activity.
Figures
Figure 1.
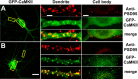
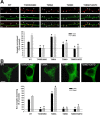
Activity-dependent translocation of GFP-CaMKII to synaptic and nonsynaptic sites in neurons. Hippocampal neurons (18 DIV) transfected with GFP-CaMKII (green) were stimulated 24 h after transfection with glutamate/glycine (100 μ
m
/10 μ
m
) for 5 min. Neurons were immediately fixed, immunostained for PSD95 (red), and imaged with confocal microscopy. A, GFP-CaMKII fluorescence is primarily diffuse in the soma (right) and dendrites (middle) of nonstimulated neurons, with occasional colocalization with PSD95 (synaptic sites) immunostaining. B, Glutamate/glycine stimulation caused a translocation of GFP-CaMKII into fluorescent puncta in both the soma and dendrites. GFP-CaMKII fluorescence in the distal dendrites (middle) appears localized primarily to PSD95-positive sites (synapses), whereas CaMKII fluorescence in the soma and initial segments of the dendrite do not (right). Images of the entire neuron (left) and cropped dendrites (middle) represent maximum projections from z-stacks, whereas those of cell bodies (right) represent a single optical slice (1.0 μm) from their center. Scale bars: (in B) left, 25 μm; (in A) middle, 3 μm; (in A) right, 5 μm.
Figure 2.
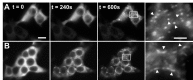
Ionomycin-induced GFP-CaMKII translocation in HEK293 cells. GFP-CaMKII was transiently transfected into HEK293 cells in the absence (A) or presence (B) of the NR2B subunit of the NMDA receptor. A, During ionomycin stimulation in HBSS (25 m
m
HEPES, pH 7.4, 2 m
m
CaCl2), the initially diffuse fluorescence became progressively punctate throughout the cytosol during 5 min of stimulation. Arrowheads in the right panel point to the spherical puncta. B, In contrast, in cells cotransfected with NR2B, the GFP-CaMKII fluorescence translocated to perinuclear endoplasmic reticulum regions, in which overexpressed NR2B subunits accumulate. Arrowheads in the right panel point to the elongated (rather than spherical) puncta. Scale bars, (in A) 6 μm. These images are representative of 8-12 experiments. To fully appreciate the difference in the two kinds of puncta observed, it is useful to look at the complete sequences of images available as movies (supplemental Fig. 2-movie1.mov and Fig. 2-movie2.mov, available at
as
supplemental material
).
Figure 3.
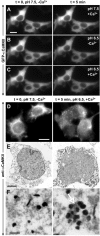
Ionomycin-induced GFP-CaMKII translocation in HEK293 cells is regulated by pH. GFP-CaMKII fluorescence was imaged following sequential ionomycin treatments in a nigericin-based extracellular buffer designed to clamp pHi at 7.5 or 6.5 as indicated. Ionomycin/Ca2+ treatment induced translocation of GFP-CaMKII at pH 6.5, as in C, but ionomycin treatment at pH 7.5 in the presence of Ca2+ (A) or at pH 6.5 in the absence of Ca2+ (B) did not induce translocation. In B and C (left), the cells were reequilibrated in nigericin/low-Ca2+/pH 7.5 buffer for 5 min before the solution changes shown in the right panels. D, Non-GFP-tagged CaMKII also translocates into clusters as revealed by immunocytochemistry for α CaMKII (right). Scale bars, (in A, D) 6 μm. E, F, Electron microscopic images of GFP-CaMKII clusters detected with anti-αCaMKII and immunogold labeling. In stimulated cells (right), groups of gold particles surround electron-dense material (arrow), whereas the gold particles are dispersed in nonstimulated cells (left) and are not associated with electron-dense material. Scale bars: E, 3 μm; F, 250 nm.
Figure 4.
Reversibility of GFP-CaMKII translocation in HEK293 cells. GFP-CaMKII translocation was induced using our standard Ca2+/ionomycin-pH drop protocol: a, prewash with nigericin-based extracellular solution, pH 7.5, without ionomycin (Time-0 image); b, switch to the same solution with ionomycin/CaCl2 for 2 min; c, switch to the same solution but at pH 6.5. A, To assess reversibility, ionomycin/Ca2+ was replaced with 5 m
m
BAPTA after punctate fluorescence peaked (240 s). Under these conditions, clusters disappeared within 2-5 min (20 experiments). B, Reversing the pH back to 7.5, after CaMKII translocation in the presence of Ca2+/ionomycin, produced little change in the punctate appearance of GFP-CaMKII (10 experiments), indicating that the reversibility is primarily dependent on the removal of Ca2+. Note that the fluorescence intensity rose slightly between Bc and Bd, likely a result of the pH sensitivity of GFP emission. Scale bar, (in Ba) 8 μm. a-e on the timeline correspond to a-e on the images. The complete sequence of images from A is available as a supplemental movie (supplemental Fig. 4-movie4.mov, available at
as
supplemental material
).
Figure 5.
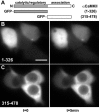
Self-association requires both catalytic and association domains. A, Schematic diagram of αCaMKII (1-478), with the catalytic (1-326) and association domains (315-478) illustrated. Cells were imaged using the standard Ca2+/ionomycin-pH drop protocol (Fig. 4_a-c_), with example frames shown for 0 min (left) and 5 min (right). B, Cells transfected with GFP-1-326-CaMKII (catalytic/autoregulatory domain). C, Cells transfected with GFP-315-478-CaMKII (association domain). Note the absence of fluorescent clusters. Scale bar, (in B) 10 μm.
Figure 6.
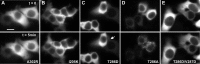
Self-association requires CaM binding, involves interactions between the autoregulatory and catalytic domains, and is regulated by Thr286 autophosphorylation. Cells were stimulated as described in Figure 4_a-c_, with example frames shown for 0 min (top) and 5 min (bottom) for Ala302Arg (A), Ile205Lys (B), Thr286Asp (C), Thr286Ala (D), and Thr286Val287/Asp286Asp287 (E). The arrow in C indicates a rare example of an HEK293 cell exhibiting clusters with Thr286Asp mutant. Scale bar, (in A) 10 μm
Figure 7.
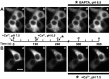
Quantification of self-association capacity by CaMKII mutants. A, Examples of confocal images (0.5-μm-thick optical slice; top) from HEK293 cells fixed after stimulation as described in Figure 4. Scale bar, 10 μm. The bottom panels show the clusters detected by our morphometric analysis (see Materials and Methods). B, Quantification of the clustering from A (n = 40 for each construct). Clustering factor, Total fluorescence inside the clusters/total fluorescence inside the cell. The asterisk indicates one-way ANOVA (F test; p < 0.001), followed by a Dunnett's test (p < 0.001) compared with control (stimulation without Ca2+). The plus sign indicates a significant difference (p < 0.001) from every condition.
Figure 8.
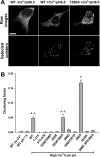
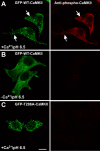
Autophosphorylation of CaMKII during self-association. HEK293 cells, stimulated as in Figure 4_a-c_, were fixed and immunostained for phospho-Thr286-CaMKII. A, GFP-WT-CaMKII clusters are autophosphorylated at Thr286 (arrows). B, In the absence of Ca2+, GFP-WT-CaMKII is not autophosphorylated at Thr286. C, GFP-Thr286A-CaMKII clusters are not immunoreactive to the anti-phospho-Thr286 antibody, showing its specificity. Scale bar, (in C) 10 μm.
Figure 9.
Role of CaMKII self-association in its activity-dependent translocation in hippocampal neurons. Hippocampal neurons (14 DIV) transfected with WT or mutated GFP-α-CaMKII were stimulated with glutamate/glycine (100 μ
m
/10 μ
m
) for 1 or 5 min and immediately fixed. A, Postsynaptic translocation of GFP-WT-CaMKII and mutants. The top panels show representative confocal images of dendrites from neurons expressing different GFP-CaMKII constructs (green; middle), which were treated for 1 min and immunostained for PSD95 (red; top). Arrowheads point to synapses. Scale bar, 3 μm. The histogram shows mean (±SEM; n = 40 synapses per construct) synaptic fluorescence intensities over the total intensity of the corresponding dendrite, relative to nonstimulated control (see Materials and Methods). B, Nonsynaptic translocation of GFP-WT-CaMKII and mutants. The top panels show confocal optical slices (0.7 μm) through the middle of neuronal cell bodies of stimulated (5 min) neurons. Scale bar, 5 μm. The histogram shows the mean (±SEM; n = 10 neurons per construct) degree of GFP-CaMKII clustering in stimulated neurons relative to nonstimulated controls (see Materials and Methods). Asterisks in A and B indicate a statistical difference (p > 0.001; one-way ANOVA followed by a Dunnett's test; p > 0.001) compared with WT for each treatment (1 or 5 min). To sample from a larger set of neurons (n > 100), we also designed a semiquantitative analysis, which yielded similar conclusions (see supplemental Fig. 2, available at
as
supplemental material
).
Figure 10.
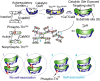
Working model for CaMKII self-association. Top, Left, Illustration of one catalytic domain, the location of key amino acids (yellow circles), and the intramolecular contacts formed between the autoregulatory and the catalytic cleft in an inactive subunit of a CaMKII holoenzyme. Top, Right, The first step in the self-association reaction, as well as enzyme activation, is Ca2+/CaM binding, which releases the autoregulatory domain to expose the catalytic domain, the substrate site, and presumably the targeting site. Middle, Left, Holoenzyme with a mix of inactive (white), Ca2+-CaM activated (green), and Thr286 autophosphorylated (red) subunits. Middle, Self-association involves binding between activated subunits from two separate holoenzymes, under the regulation by intracellular pH, which is hypothesized to affect displacement of the autoinhibitory domain from the catalytic pocket (middle, right). Bottom panels (circles 1-4) represent hypothesized interactions that activated subunits undergo, depending on the various possible combinations of Thr286 phosphorylation (red circles). 1, 2, Autophosphorylated Thr286 cannot interact with the targeting site, preventing association. 3, 4, Thr286 region of activated subunits (but not phosphorylated) can interact with the targeting site of another subunit, whether (3) or not (4) the latter is phosphorylated. Those potential interactions are exemplified in the cluster of CaMKII (see 1-4 in the middle).
Similar articles
- Transition from reversible to persistent binding of CaMKII to postsynaptic sites and NR2B.
Bayer KU, LeBel E, McDonald GL, O'Leary H, Schulman H, De Koninck P. Bayer KU, et al. J Neurosci. 2006 Jan 25;26(4):1164-74. doi: 10.1523/JNEUROSCI.3116-05.2006. J Neurosci. 2006. PMID: 16436603 Free PMC article. - Activation of dopamine D4 receptors induces synaptic translocation of Ca2+/calmodulin-dependent protein kinase II in cultured prefrontal cortical neurons.
Gu Z, Jiang Q, Yuen EY, Yan Z. Gu Z, et al. Mol Pharmacol. 2006 Mar;69(3):813-22. doi: 10.1124/mol.105.018853. Epub 2005 Dec 19. Mol Pharmacol. 2006. PMID: 16365279 - Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation.
Shen K, Meyer T. Shen K, et al. Science. 1999 Apr 2;284(5411):162-6. doi: 10.1126/science.284.5411.162. Science. 1999. PMID: 10102820 - Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function.
Hudmon A, Schulman H. Hudmon A, et al. Annu Rev Biochem. 2002;71:473-510. doi: 10.1146/annurev.biochem.71.110601.135410. Epub 2001 Nov 9. Annu Rev Biochem. 2002. PMID: 12045104 Review. - Activity-driven postsynaptic translocation of CaMKII.
Merrill MA, Chen Y, Strack S, Hell JW. Merrill MA, et al. Trends Pharmacol Sci. 2005 Dec;26(12):645-53. doi: 10.1016/j.tips.2005.10.003. Epub 2005 Oct 25. Trends Pharmacol Sci. 2005. PMID: 16253351 Review.
Cited by
- Cytoskeleton Protein EB3 Contributes to Dendritic Spines Enlargement and Enhances Their Resilience to Toxic Effects of Beta-Amyloid.
Pchitskaya E, Rakovskaya A, Chigray M, Bezprozvanny I. Pchitskaya E, et al. Int J Mol Sci. 2022 Feb 18;23(4):2274. doi: 10.3390/ijms23042274. Int J Mol Sci. 2022. PMID: 35216391 Free PMC article. - Autonomous CaMKII can promote either long-term potentiation or long-term depression, depending on the state of T305/T306 phosphorylation.
Pi HJ, Otmakhov N, Lemelin D, De Koninck P, Lisman J. Pi HJ, et al. J Neurosci. 2010 Jun 30;30(26):8704-9. doi: 10.1523/JNEUROSCI.0133-10.2010. J Neurosci. 2010. PMID: 20592192 Free PMC article. - The Cav1.2 N terminus contains a CaM kinase site that modulates channel trafficking and function.
Simms BA, Souza IA, Rehak R, Zamponi GW. Simms BA, et al. Pflugers Arch. 2015 Apr;467(4):677-86. doi: 10.1007/s00424-014-1538-7. Epub 2014 May 28. Pflugers Arch. 2015. PMID: 24862738 - Displacement of alpha-actinin from the NMDA receptor NR1 C0 domain By Ca2+/calmodulin promotes CaMKII binding.
Merrill MA, Malik Z, Akyol Z, Bartos JA, Leonard AS, Hudmon A, Shea MA, Hell JW. Merrill MA, et al. Biochemistry. 2007 Jul 24;46(29):8485-97. doi: 10.1021/bi0623025. Epub 2007 Jun 30. Biochemistry. 2007. PMID: 17602661 Free PMC article. - Effective post-insult neuroprotection by a novel Ca(2+)/ calmodulin-dependent protein kinase II (CaMKII) inhibitor.
Vest RS, O'Leary H, Coultrap SJ, Kindy MS, Bayer KU. Vest RS, et al. J Biol Chem. 2010 Jul 2;285(27):20675-82. doi: 10.1074/jbc.M109.088617. Epub 2010 Apr 27. J Biol Chem. 2010. PMID: 20424167 Free PMC article.
References
- Aronowski J, Grotta JC (1996) Ca2+/calmodulin-dependent protein kinase II in postsynaptic densities after reversible cerebral ischemia in rats. Brain Res 709: 103-110. - PubMed
- Bayer KU, Schulman H (2001) Regulation of signal transduction by protein targeting: the case for CaMKII. Biochem Biophys Res Commun 289: 917-923. - PubMed
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H (2001) Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature 411: 801-805. - PubMed
- Chesler M, Kaila K (1992) Modulation of pH by neuronal activity. Trends Neurosci 15: 396-402. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous