Disruption of the paternal necdin gene diminishes TrkA signaling for sensory neuron survival - PubMed (original) (raw)
Disruption of the paternal necdin gene diminishes TrkA signaling for sensory neuron survival
Ken-ichiro Kuwako et al. J Neurosci. 2005.
Abstract
Necdin is a multifunctional signaling protein that stabilizes terminal differentiation of postmitotic neurons. The human necdin gene in chromosome 15q11-q12 is maternally imprinted, paternally transcribed, and not expressed in Prader-Willi syndrome, a human genomic imprinting-associated neurodevelopmental disorder. Although necdin-deficient mice display several abnormal phenotypes reminiscent of this syndrome, little is known about molecular mechanisms that lead to the neurodevelopmental defects. Here, we demonstrate that paternally expressed necdin is required for physiological development of nerve growth factor (NGF)-dependent sensory neurons. Mouse embryos defective in the paternal necdin allele displayed absent necdin expression in the dorsal root ganglia, in which the tropomyosin-related kinase A (TrkA) receptor tyrosine kinase and the p75 neurotrophin receptor were expressed in a normal manner. Necdin interacted with both TrkA and p75 to facilitate the association between these receptors. NGF-induced phosphorylation of TrkA and mitogen-activated protein kinase was significantly diminished in the necdin-null sensory ganglia. Furthermore, the mice lacking the paternal necdin allele displayed augmented apoptosis in the sensory ganglia in vivo and had a reduced population of substance P-containing neurons. These mutant mice showed significantly high tolerance to thermal pain, which is often seen in individuals with Prader-Willi syndrome. These results suggest that paternally expressed necdin facilitates TrkA signaling to promote the survival of NGF-dependent nociceptive neurons.
Figures
Figure 1.
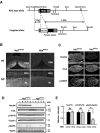
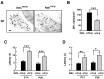
Necdin expression is absent in DRGs of mutant mice lacking the paternal necdin gene. A, The restriction map of mouse Ndn locus. A 7 kb Ndn sequence containing coding (black) and 3′,5′ noncoding (hatched) regions was subcloned into the targeting vector. The 1.5 kb expression unit containing the pgk promoter with Neor gene (Pgk1/neor), polyadenylation signal [Poly(A) signal], was inserted to disrupt the Ndn coding sequence. The vector was introduced to TT2 cells for homologous recombination as described in Materials and Methods. B, Absence of necdin expression in the brain and spinal cord of mice deficient in the paternal necdin gene. Frozen sections of the hypothalamus and spinal cord from E13.5 wild-type (Ndn+m/+p) and necdin-deficient (Ndn+m/-p) littermates were immunostained for necdin. HT, Hypothalamus; SC, spinal cord; DBB, diagonal band of Broca; ML, mantle layer. Scale bars: HT, 50 μm; SC, 100 μm. C, Expression patterns of TrkA and p75NTR in necdin-deficient DRGs. Frozen sections of cervical DRGs from E12.5 wild-type (Ndn+m/+p) and necdin-deficient (Ndn+m/-p) littermates were immunostained for necdin, TrkA, and p75NTR. Scale bar, 50 μm. D, E, Western blot analysis of necdin, TrkA, p75NTR, and phosphorylated MAPK. Equal amounts of DRG lysates from E12.5 embryos were analyzed by Western blotting. Each lane represents pooled DRGs from a single embryo. Molecular sizes in kilodaltons are shown to the right. E, Signal intensities of TrkA, p75NTR, and pMAPK shown in D were normalized to those of tubulin (TB) and MAPK (mean ± SEM; n = 5 for each protein). *p < 0.05. NS, Not significant (_p_ > 0.05).
Figure 2.
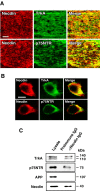
Necdin is coexpressed with TrkA or p75NTR in developing DRGs. A, B, Double immunostaining for necdin and TrkA or p75NTR. Frozen DRG sections of E13.5 mouse embryo were double immunostained for necdin with antibody GN1 (red) and TrkA (or p75NTR) (green), and two images were merged (yellow). Scale bars: A, 50 μm; B, 10 μm. C, Immunoaffinity assay for endogenous necdin-interacting proteins. Tissue lysate (1 mg) of the DRG and spinal cord from E13.5 embryos was applied to the immunoaffinity columns with anti-necdin IgG (NC243) (αNecdin IgG) and control preimmune IgG (Preimmune IgG). The eluates were analyzed by Western blotting for endogenous complexes of necdin with TrkA, p75NTR, and APP. Lysate, Tissue lysate (30 μg).
Figure 3.
Necdin interacts with both TrkA and p75NTR. A, B, Immunoprecipitation assay for interactions of necdin with TrkA and p75NTR. Lysates of HEK293A cells transfected with expression vectors for FLAG-TrkA, FLAG-p75NTR, FLAG-APP, and necdin were immunoprecipitated (IP) with anti-FLAG antibody M2 (αFLAG) and immunoblotted (IB) with anti-necdin antibody NC243 (αNecdin) (A, top panel). Conversely, the lysates were immunoprecipitated with antibody NC243 and immunoblotted with antibody M2 (B, top panel). Expressed proteins in cell lysates are shown in the bottom panels. C, Necdin-enhanced association between TrkA and p75NTR. Lysates of cells transfected with expression vectors for HA-p75NTR (4 μg), FLAG-TrkA (4.5 μg), and necdin (0.1 and 0.5 μg) were immunoprecipitated with antibody M2 and immunoblotted with anti-p75NTR antibody (top panel). D, Interactions of necdin with TrkA mutants. Lysates of cDNA-transfected cells expressing necdin, FLAG-tagged wild-type (WT) TrkA, and the mutants (K547A, Y499A, Y794A) were immunoprecipitated as in A. Phosphorylated Tyr499 in rat TrkA was analyzed using the antibody against phospho-human TrkA (Tyr490) (αpTrkA) (D, second panel from the top).
Figure 4.
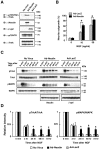
Necdin enhances NGF/TrkA signaling in PC12 cells. A, Coimmunoprecipitation assay for the association between TrkA and p75NTR. PC12 cells infected with recombinant adenoviruses expressing necdin (Ad-Necdin) and β-galactosidase (Ad-LacZ) were treated with NGF (50 ng/ml) for 38 h. Cell lysates (400 μg) were immunoprecipitated (IP) with mouse anti-p75NTR antibody MC192 (αp75NTRm) and immunoblotted (IB) with anti-TrkA antibody (top panel). Expressed proteins in cell lysates are shown in the bottom panels. B, Neurite outgrowth assay. Ad-Necdin- and Ad-LacZ-infected PC12 cells were treated with NGF for 38 h, and cells bearing extended neurites were counted (mean ± SEM; n = 4; p < 0.005). C, D, Western blot analysis of phosphorylated forms of TrkA and MAPK. PC12 cells were infected with Ad-Necdin and Ad-LacZ, incubated for 36 h, and treated with NGF (10 ng/ml) for the indicated durations. C, Phosphorylated proteins (pTrkA, pMAPK) were analyzed by Western blotting using phosphoprotein-specific antibodies. Similar data were obtained from two more sets of Western blot analysis. D, Signal intensities of pTrkA and pMAPK were normalized to those of respective total proteins including unphosphorylated forms. The values relative to those of the maximum intensities (=1) at 5 min are presented (mean ± SEM; n = 3). *p < 0.005 (compared with the controls treated with Ad-LacZ).
Figure 5.
NGF/TrkA signaling is diminished in necdin-null DRG explants. A, B, Neurite outgrowth assay. DRG explants were prepared from E13.5 embryos, treated with NGF for 16 h, and analyzed by phase-contrast microscopy. A, Typical images of NGF-treated DRG explants. B, The neurite length of the explants was measured after NGF treatment for 16 h (explants examined: +m/+p, n = 105; +m/-p, n = 102). Scale bar: A, 200 μm. C, D, Western blot analysis of phosphorylated forms of TrkA and MAPK. DRG explants from E13.5 littermates were cultured in the presence of NGF (20 ng/ml) for the indicated durations. Phosphorylated proteins (pTrkA, pMAPK) were analyzed by Western blotting using phosphoprotein-specific antibodies. The values relative to those of the wild-type controls (=1) at 15 min were presented (mean ± SEM; n = 4). B, D, Graphs show mean ± SEM. *p < 0.01; **p < 0.005; ***p < 0.001.
Figure 6.
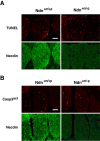
Apoptosis is augmented in necdin-deficient embryonic DRGs in vivo. A, TUNEL assay. Frozen sections of cervical DRGs from wild-type (Ndn+m/+p) and necdin-deficient (Ndn+m/-p) littermates at E12.5 were double stained for TUNEL and necdin with antibody NC243. B, Immunohistochemistry for activated capase-3. Frozen sections of cervical DRGs from wild-type and necdin-deficient littermates at E12.5 were double immunostained for activated caspase-3 (Casp3act) with rabbit cleaved caspase-3 end-specific antibody and for necdin with guinea pig anti-necdin antibody GN1. Scale bars, 50 μm. Quantitative data for A and B are given in Results.
Figure 7.
Development of NGF-dependent DRG neurons is impaired in necdin-deficient mice. A, B, Immunohistochemistry for substance P neurons in DRGs. A, Frozen sections of mouse DRGs from wild-type (Ndn+m/+p) and necdin-deficient (Ndn+m/-p) littermates at P0 were immunostained for substance P (SP). Scale bar: A, 50 μm. B, Substance P-positive (SP+) cells (A, arrowheads) in each DRG section were counted (DRG slices examined: +m/+p, n = 108; +m/-p, n = 98). C, D, Behavioral analyses of heat-induced pain. Wild-type and necdin-deficient littermates at P14 and P28 were subjected to hotplate (C) and tail-flick (D) tests to measure the latencies (animals examined: +m/+p, n = 7; +m/-p, n = 6). Similar data were obtained from four more groups of littermates. B-D, Graphs show mean ± SEM. *p < 0.05; **p < 0.002; ***p < 0.001.
Similar articles
- Necdin: A purposive integrator of molecular interaction networks for mammalian neuron vitality.
Yoshikawa K. Yoshikawa K. Genes Cells. 2021 Sep;26(9):641-683. doi: 10.1111/gtc.12884. Epub 2021 Aug 2. Genes Cells. 2021. PMID: 34338396 Free PMC article. Review. - Necdin and neurotrophin receptors: interactors of relevance for neuronal resistance to oxidant stress.
Ingraham CA, Wertalik L, Schor NF. Ingraham CA, et al. Pediatr Res. 2011 Apr;69(4):279-84. doi: 10.1203/PDR.0b013e31820a5773. Pediatr Res. 2011. PMID: 21150695 Free PMC article. - Sensory defects in Necdin deficient mice result from a loss of sensory neurons correlated within an increase of developmental programmed cell death.
Andrieu D, Meziane H, Marly F, Angelats C, Fernandez PA, Muscatelli F. Andrieu D, et al. BMC Dev Biol. 2006 Nov 20;6:56. doi: 10.1186/1471-213X-6-56. BMC Dev Biol. 2006. PMID: 17116257 Free PMC article. - Necdin downregulates CDC2 expression to attenuate neuronal apoptosis.
Kurita M, Kuwajima T, Nishimura I, Yoshikawa K. Kurita M, et al. J Neurosci. 2006 Nov 15;26(46):12003-13. doi: 10.1523/JNEUROSCI.3002-06.2006. J Neurosci. 2006. PMID: 17108174 Free PMC article. - [Molecular mechanisms of differentiation and death of human neurons: with special reference to necdin and APP].
Yoshikawa K. Yoshikawa K. Nihon Shinkei Seishin Yakurigaku Zasshi. 2000 Oct;20(4):155-9. Nihon Shinkei Seishin Yakurigaku Zasshi. 2000. PMID: 11215400 Review. Japanese.
Cited by
- CNTNAP2 intracellular domain (CICD) generated by γ-secretase cleavage improves autism-related behaviors.
Zhang J, Cai F, Lu R, Xing X, Xu L, Wu K, Gong Z, Zhang Q, Zhang Y, Xing M, Song W, Li JD. Zhang J, et al. Signal Transduct Target Ther. 2023 Jun 5;8(1):219. doi: 10.1038/s41392-023-01431-6. Signal Transduct Target Ther. 2023. PMID: 37271769 Free PMC article. - Necdin: A purposive integrator of molecular interaction networks for mammalian neuron vitality.
Yoshikawa K. Yoshikawa K. Genes Cells. 2021 Sep;26(9):641-683. doi: 10.1111/gtc.12884. Epub 2021 Aug 2. Genes Cells. 2021. PMID: 34338396 Free PMC article. Review. - Genetic dissection identifies Necdin as a driver gene in a mouse model of paternal 15q duplications.
Tamada K, Fukumoto K, Toya T, Nakai N, Awasthi JR, Tanaka S, Okabe S, Spitz F, Saitow F, Suzuki H, Takumi T. Tamada K, et al. Nat Commun. 2021 Jul 1;12(1):4056. doi: 10.1038/s41467-021-24359-3. Nat Commun. 2021. PMID: 34210967 Free PMC article. - Hypothalamic neuropeptides and neurocircuitries in Prader Willi syndrome.
Correa-da-Silva F, Fliers E, Swaab DF, Yi CX. Correa-da-Silva F, et al. J Neuroendocrinol. 2021 Jul;33(7):e12994. doi: 10.1111/jne.12994. Epub 2021 Jun 22. J Neuroendocrinol. 2021. PMID: 34156126 Free PMC article. Review. - A Comprehensive Review of Genetically Engineered Mouse Models for Prader-Willi Syndrome Research.
Kummerfeld DM, Raabe CA, Brosius J, Mo D, Skryabin BV, Rozhdestvensky TS. Kummerfeld DM, et al. Int J Mol Sci. 2021 Mar 31;22(7):3613. doi: 10.3390/ijms22073613. Int J Mol Sci. 2021. PMID: 33807162 Free PMC article. Review.
References
- Aizawa T, Maruyama K, Kondo H, Yoshikawa K (1992) Expression of necdin, an embryonal carcinoma-derived nuclear protein, in developing mouse brain. Brain Res Dev Brain Res 68: 265-274. - PubMed
- Andrieu D, Watrin F, Niinobe M, Yoshikawa K, Muscatelli F, Fernandez PA (2003) Expression of the Prader-Willi gene Necdin during mouse nervous system development correlates with neuronal differentiation and p75NTR expression. Gene Expr Patterns 3: 761-765. - PubMed
- Bibel M, Barde YA (2000) Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev 14: 2919-2937. - PubMed
- Brandt BR, Rosen I (1998) Impaired peripheral somatosensory function in children with Prader-Willi syndrome. Neuropediatrics 29: 124-126. - PubMed
- Chao MV (2003) Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4: 299-309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials