Proliferation of estrogen receptor-alpha-positive mammary epithelial cells is restrained by transforming growth factor-beta1 in adult mice - PubMed (original) (raw)
Comparative Study
Proliferation of estrogen receptor-alpha-positive mammary epithelial cells is restrained by transforming growth factor-beta1 in adult mice
Kenneth B R Ewan et al. Am J Pathol. 2005 Aug.
Abstract
Transforming growth factor (TGF)-beta1 is a potent inhibitor of mammary epithelial proliferation. In human breast, estrogen receptor (ER)-alpha cells rarely co-localize with markers of proliferation, but their increased frequency correlates with breast cancer risk. To determine whether TGF-beta1 is necessary for the quiescence of ER-alpha-positive populations, we examined mouse mammary epithelial glands at estrus. Approximately 35% of epithelial cells showed TGF-beta1 activation, which co-localized with nuclear receptor-phosphorylated Smad 2/3, indicating that TGF-beta signaling is autocrine. Nuclear Smad co-localized with nuclear ER-alpha. To test whether TGF-beta inhibits proliferation, we examined genetically engineered mice with different levels of TGF-beta1. ER-alpha co-localization with markers of proliferation (ie, Ki-67 or bromodeoxyuridine) at estrus was significantly increased in the mammary glands of Tgf beta1 C57/bl/129SV heterozygote mice. This relationship was maintained after pregnancy but was absent at puberty. Conversely, mammary epithelial expression of constitutively active TGF-beta1 via the MMTV promoter suppressed proliferation of ER-alpha-positive cells. Thus, TGF-beta1 activation functionally restrains ER-alpha-positive cells from proliferating in adult mammary gland. Accordingly, we propose that TGF-beta1 dysregulation may promote proliferation of ER-alpha-positive cells associated with breast cancer risk in humans.
Figures
Figure 1
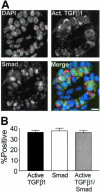
Nuclear R-Smad protein co-localizes in mammary epithelial cells that are positive for active TGF-β1. A: Individual channel images of DAPI-stained nuclei (DAPI), R-Smad, and TGF-β1 immunostaining in a transverse section of a duct. Merged image shows R-Smad immunoreactivity as green and active TGF-β1 as red. B: Active TGF-β1 and nuclear R-Smad are markers of the same cell population in the mammary epithelium at estrus. Shown are means ± SEM from three C57BL/6–129SV mixed background animals. At least 250 cells were scored per animal. Scale bar, 20 μm.
Figure 2
Subpopulations of the mammary epithelium of the C57BL/6–129SV mixed background mouse at estrus. A: Example of dual-immunofluorescence localization of ER-α and R-Smad immunoreactivity shows the gray scale individual images of DAPI-stained nuclei, ER-α, R-Smad, and a merged color image showing ER-α immunoreactivity as green and nuclear Smad as red which makes nuclei positive for both appear yellow/orange. Column graph shows mean co-localization frequency ± SEM (n = 3). B: Gray scale images of DAPI-stained nuclei, PR, and active TGF-β1 immunostaining in ductal epithelium. The merged color image shows that most PR-positive cells (green nuclei) are also positive for cytoplasmic active TGF-β1 (red). Column graph shows mean co-localization frequency ± SEM (n = 3). C: Individual channels and merged image of ER-α and PR immunoreactivity in a transverse section of a duct. ER-α-positive cells all exhibit PR and the nuclei appear orange in the merged image. Column graph shows mean co-localization frequency ± SEM (n = 3). Scale bar, 20 μm.
Figure 3
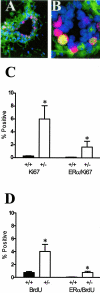
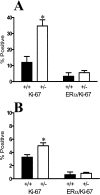
TGF-β1 depletion results in increased frequency of ER-α-positive mammary epithelial cells in cycle. A: Dual-immunofluorescence localization of ER-α (green; nonnuclear staining is nonspecific) and Ki67 (red) in mammary epithelium. Nuclei are counterstained with DAPI (blue). B: Dual-immunofluorescence localization of ER-α and Ki67, as in A, of higher magnification of a mammary duct with an example of double-labeled nucleus (yellow). C: Ki-67 and ER-α/Ki-67 co-localization frequency in mammary epithelium of nulliparous Tgf_β_1 heterozygote (+/− in figure) and Tgf_β_1 wild-type (+/+) C57BL/6–129SV mice at estrus. Three animals per genotype and at least 300 cells per animal were scored for presence of ER-α and Ki-67 immunoreactivity. Asterisks indicate significant difference from Tgf_β_1 wild-type mean frequency (P < 0.01; _t_-test). D: BrdU and ER-α/BrdU co-localization frequency in mammary epithelium of the same nulliparous Tgf_β_1 heterozygote (+/−) and Tgf_β_1 wild-type (+/+) C57BL/6–129SV mice at estrus. Asterisks indicate significant difference from Tgf_β_1 wild-type mean frequency (P < 0.01; _t_-test).
Figure 4
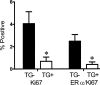
Expression of constitutively active TGF-β1 results in decreased mammary epithelial co-localization of ER-α and proliferation. Ki-67 and ER-α/Ki-67 co-localization frequency in mammary epithelium of 12-week-old nulliparous MMTV-TGF_β_223_–_225 FvB transgenic mice (TG+) and age-matched wild-type mice (TG−) mice at estrus. Asterisks indicate significant difference from Tgf_β_1 wild-type mean frequency (P < 0.05; _t_-test).
Figure 5
TGF-β1 depletion results in increased frequency of ER-α-positive mammary epithelial cells in cycle in parous mice. Ki-67 and ER-α/Ki-67 co-localization frequency in mammary epithelium of parous Tgf_β_1 heterozygote (+/−) and Tgf_β_1 wild-type (+/+) C57BL/6–129SV mice at estrus. These animals were sacrificed 3 weeks after weaning. Four animals per genotype and at least 250 cells per animal were scored for presence of ER-α and Ki-67 immunoreactivity. Asterisk indicates significant difference from Tgf_β_1 wild-type mean frequency (P < 0.05; _t_-test).
Figure 6
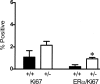
TGF-β1 depletion in pubertal glands results in increased frequency of cells in cycle but not in proliferating ER-α-positive mammary epithelial cells. A: Ki-67 and ER-α/Ki-67 co-localization frequency in endbud mammary epithelium of TGF-β1 heterozygote (+/−) and TGF-β1 wild-type (+/+) C57BL/6–129SV mice at 6 weeks of age. Three animals per genotype and at least 300 cells per animal were scored for presence of ER-α and Ki-67 immunoreactivity. B: Ki-67 and ER-α/Ki-67 co-localization frequency in the mammary epithelium of subtending ducts in the same tissues. Asterisks indicate significant difference from Tgf_β_1 wild-type mean frequency (P < 0.01; _t_-test).
Similar articles
- Latent transforming growth factor-beta activation in mammary gland: regulation by ovarian hormones affects ductal and alveolar proliferation.
Ewan KB, Shyamala G, Ravani SA, Tang Y, Akhurst R, Wakefield L, Barcellos-Hoff MH. Ewan KB, et al. Am J Pathol. 2002 Jun;160(6):2081-93. doi: 10.1016/s0002-9440(10)61158-3. Am J Pathol. 2002. PMID: 12057913 Free PMC article. - Alterations of Smad signaling in human breast carcinoma are associated with poor outcome: a tissue microarray study.
Xie W, Mertens JC, Reiss DJ, Rimm DL, Camp RL, Haffty BG, Reiss M. Xie W, et al. Cancer Res. 2002 Jan 15;62(2):497-505. Cancer Res. 2002. PMID: 11809701 - TGF beta regulation of cell proliferation.
Moses HL, Arteaga CL, Alexandrow MG, Dagnino L, Kawabata M, Pierce DF Jr, Serra R. Moses HL, et al. Princess Takamatsu Symp. 1994;24:250-63. Princess Takamatsu Symp. 1994. PMID: 8983080 Review. - Hormone-sensing mammary epithelial progenitors: emerging identity and hormonal regulation.
Tarulli GA, Laven-Law G, Shakya R, Tilley WD, Hickey TE. Tarulli GA, et al. J Mammary Gland Biol Neoplasia. 2015 Jun;20(1-2):75-91. doi: 10.1007/s10911-015-9344-1. Epub 2015 Sep 21. J Mammary Gland Biol Neoplasia. 2015. PMID: 26390871 Review.
Cited by
- miR-30a-3p Regulates Autophagy in the Involution of Mice Mammary Glands.
Tian L, Guo S, Zhao Z, Chen Y, Wang C, Li Q, Li Y. Tian L, et al. Int J Mol Sci. 2023 Sep 20;24(18):14352. doi: 10.3390/ijms241814352. Int J Mol Sci. 2023. PMID: 37762652 Free PMC article. - A multiscale statistical mechanical framework integrates biophysical and genomic data to assemble cancer networks.
AlQuraishi M, Koytiger G, Jenney A, MacBeath G, Sorger PK. AlQuraishi M, et al. Nat Genet. 2014 Dec;46(12):1363-1371. doi: 10.1038/ng.3138. Epub 2014 Nov 2. Nat Genet. 2014. PMID: 25362484 Free PMC article. - Transcriptome analysis of the hormone-sensing cells in mammary epithelial reveals dynamic changes in early pregnancy.
De Silva D, Kunasegaran K, Ghosh S, Pietersen AM. De Silva D, et al. BMC Dev Biol. 2015 Jan 27;15:7. doi: 10.1186/s12861-015-0058-9. BMC Dev Biol. 2015. PMID: 25623114 Free PMC article. - Heterozygosity for TGF β1 -509C/T Polymorphism is associated with risk for breast cancer in South Indian population.
Vinod C, Jyothy A, Vijay Kumar M, Raghu Raman R, Nallari P, Venkateshwari A. Vinod C, et al. Tumour Biol. 2013 Feb;34(1):99-105. doi: 10.1007/s13277-012-0516-y. Epub 2012 Sep 22. Tumour Biol. 2013. PMID: 23001908 - ER and PR signaling nodes during mammary gland development.
Tanos T, Rojo L, Echeverria P, Brisken C. Tanos T, et al. Breast Cancer Res. 2012 Jul 19;14(4):210. doi: 10.1186/bcr3166. Breast Cancer Res. 2012. PMID: 22809143 Free PMC article. Review.
References
- Daniel CW, Robinson SD. Regulation of mammary growth and function by TGF-β. Mol Reprod Dev. 1992;32:145–151. - PubMed
- Derynck R, Ackhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. - PubMed
- Wakefield L, Colletta AA, McCune BK, Sporn MB. Roles for transforming growth factors-β in the genesis, prevention and treatment of breast cancer. Dickson RB, Lippman ME, editors. Boston: Kluwer Academic Publishers,; Genes, Oncogens, and HormonesAdvances in Cellular and Molecular Biology of Breast Cancer. 1991:pp 97–136. - PubMed
- Smith GH. TGF-β and functional differentiation. J Mammary Gland Biol Neoplasia. 1996;1:343–352. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous