Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy - PubMed (original) (raw)
Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy
Mohamed Al-Shabrawey et al. Am J Pathol. 2005 Aug.
Abstract
Because oxidative stress has been strongly implicated in up-regulation of vascular endothelial growth factor (VEGF) expression in ischemic retinopathy, we evaluated the role of NAD(P)H oxidase in causing VEGF overexpression and retinal neovascularization. Dihydroethidium imaging analyses showed increased superoxide formation in areas of retinal neovascularization associated with relative retinal hypoxia in a mouse model for oxygen-induced retinopathy. The effect of hypoxia in stimulating superoxide formation in retinal vascular endothelial cells was confirmed by in vitro chemiluminescence assays. The superoxide formation was blocked by specific inhibitors of NAD(P)H oxidase activity (apocynin, gp91ds-tat) indicating that NAD(P)H oxidase is a major source of superoxide formation. Western blot and immunolocalization analyses showed that retinal ischemia increased expression of the NAD(P)H oxidase catalytic subunit gp91phox, which localized primarily within vascular endothelial cells. Treatment of mice with apocynin blocked ischemia-induced increases in oxidative stress, normalized VEGF expression, and prevented retinal neovascularization. Apocynin and gp91ds-tat also blocked the action of hypoxia in causing increased VEGF expression in vitro, confirming the specific role of NAD(P)H oxidase in hypoxia-induced increases in VEGF expression. In conclusion, NAD(P)H oxidase activity is required for hypoxia-stimulated increases in VEGF expression and retinal neovascularization. Inhibition of NAD(P)H oxidase offers a new therapeutic target for the treatment of retinopathy.
Figures
Figure 1
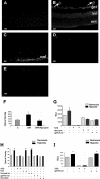
Superoxide generation in mouse retina was assessed in vitro by using the oxidative fluorescent dye DHE in retinal sections from mice maintained in room air (A) or exposed to hyperoxia for 5 days and returned to room air for 5 days (B–E). There was a marked increase in fluorescence intensity in the retinas and new vessels of the ischemic retina (arrows, B) as well as within the ganglion cell layer (gcl), inner nuclear layer (inl), and outer nuclear layer (onl). DHE reaction was reduced in retinal sections treated with gp91ds-tat (C), apocynin (D), or PEG-SOD (E). DCF assay of reactive oxygen species production in retina (F) showed a significant increase in retina during OIR that was inhibited in apocynin-treated mice (*P = < 0.05, n = 5). Chemiluminescence assay of superoxide production by retinal endothelial cells (G) or rat Muller cells (I) exposed to hypoxia (1% O2, 6 hours) shows a significant increase in the generation of superoxide anion. Treatment with the NAD(P)H oxidase inhibitors apocynin or the gp91ds-tat blocking peptide prevented the hypoxia-induced increase in endothelial cells but not in rat Muller cells. The signal was completely eliminated by treatment with PEG-SOD (*P = < 0.05, n = 4; RLU, relative light unit). DHE assay of superoxide production by endothelial cells (H) showed a significant increase in hypoxia-treated cells that was blocked by PEG-SOD, gp91ds-tat, and apocynin (*P = < 0.05, n = 5). Scale bars, 20 μm.
Figure 2
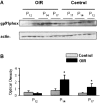
Immunoblot analysis of gp91phox expression at P12, P14, and P17. A: Levels of gp91phox are markedly increased at P14 and P17 in retinas of mice raised in hyperoxia for 5 days and returned to room air for 5 days (OIR) as compared with age-matched controls (control) raised in normoxia. B: Results were quantified by densitometry (*P < 0.05 versus age-matched control, n = 6).
Figure 3
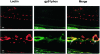
Immunohistochemical localization of gp91phox and vascular endothelial cells. Retinal sections from normoxia control (top) and ischemic retinas (bottom) were reacted with GSI lectin to label the vessels (red) and gp91phox antibody (green). Merged images show low levels of gp91phox protein in the normal retina and marked increases in the ischemic retina, especially within the proliferating vessels (yellow, arrows). Scale bars, 20 μm.
Figure 4
Immunohistochemical localization of gp91phox with vascular endothelial cells (A, B) and with retinal glia (C) at P17 in the ischemic retina. Double labeling with antibodies against CD31 (red) and gp91phox (green) shows that gp91phox co-localizes with vascular endothelial cells in the ischemic retina (yellow, arrows). Inset in A is shown at higher magnification in B. Double labeling with antibodies against GFAP (red) and gp91phox (green) shows a limited co-localization of gp91phox with retinal glia (yellow, arrows), whereas the neovascular tuft showed strong gp91phox expression (arrowheads). Scale bar, 20 μm.
Figure 5
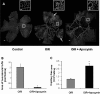
Flat-mounted retinas reacted with GSI lectin to localize neovascularization and capillary obliteration. A: Mice were maintained in hyperoxia from P7 to P12, returned to room air and treated with apocynin (10 mg/kg/day, ip) or vehicle from P12 to P17. Retina from P17 control mouse shows a normal vascular pattern. Retina from nontreated P17 hyperoxia-exposed mouse shows neovascular tufts in the mid-periphery (arrow) and a zone of capillary obliteration around the optic disk. Retina from apocynin-treated P17 mouse shows almost no neovascular tufts and a large capillary-free zone around the optic disk. B: Quantitative comparison of the area occupied by neovascular tufts in the ischemic retinas on P17 shows a significant decrease in neovascularization in the apocynin-treated mice as compared with the nontreated mice (*P < 0.05, n = 5). C: Measurement of the capillary-free zone in the ischemic retinas on P17 shows a significant decrease in revascularization of the central retina of apocynin-treated mice as compared with the nontreated mice (*P < 0.05, n = 5). Scale bars: 100 μm; 20 μm (inset).
Figure 6
Flat-mounted retinas reacted with GSI lectin to localize neovascularization and capillary obliteration. Mice were maintained in hyperoxia from P7 to P12, returned to room air for 10 days and treated with apocynin (10 mg/kg/day, ip) or vehicle for various times. Retina of a nontreated ischemic mouse retina at P22 shows capillary tufts on the surface of retina (arrows) and a small capillary-free zone around the optic disk. Middle: Retina of a P22 mouse that had been treated with apocynin from P12 to P22, demonstrating the absence of capillary tufts, but persistence of the capillary-free zone around the optic disk. Right: Retina of a P22 mouse that had been treated with apocynin from P12 to P17 and allowed to recover for 5 days until P22, demonstrating the absence of capillary tufts and almost complete revascularization of the central retina. Scale bars, 100 μm.
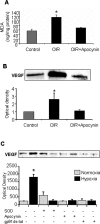
Figure 7
MDA analysis of lipid peroxidation (A) and Western blot analysis of VEGF expression (B) in mice maintained in hyperoxia from P7 to P12 and then returned to room air and treated with apocynin (10 mg/kg/day, ip) or vehicle for 2 days shows significant increases in lipid peroxidation and VEGF expression in the OIR retinas. Both effects were blocked by apocynin (*P < 0.05 versus control, n = 4 for MDA, n = 6 for Western blot). Western blot analysis of VEGF expression in retinal endothelial cells (C) exposed to normoxia (N) and hypoxia (H, 1% O2, 6 hours) shows a significant increase in VEGF expression that was completely inhibited by treatment with the NAD(P)H oxidase inhibitors apocynin (Apo), gp91ds-tat blocking peptide (BP), or PEG-SOD (*P < 0.05 versus normoxia control, n = 3).
Similar articles
- Inhibition of NAD(P)H oxidase reduces apoptosis and avascular retina in an animal model of retinopathy of prematurity.
Saito Y, Geisen P, Uppal A, Hartnett ME. Saito Y, et al. Mol Vis. 2007 Jun 12;13:840-53. Mol Vis. 2007. PMID: 17615545 Free PMC article. - NAD(P)H oxidase-dependent regulation of CCL2 production during retinal inflammation.
Zhang W, Rojas M, Lilly B, Tsai NT, Lemtalsi T, Liou GI, Caldwell RW, Caldwell RB. Zhang W, et al. Invest Ophthalmol Vis Sci. 2009 Jun;50(6):3033-40. doi: 10.1167/iovs.08-2676. Epub 2009 Feb 21. Invest Ophthalmol Vis Sci. 2009. PMID: 19234337 Free PMC article. - NO-mediated regulation of NAD(P)H oxidase by laminar shear stress in human endothelial cells.
Duerrschmidt N, Stielow C, Muller G, Pagano PJ, Morawietz H. Duerrschmidt N, et al. J Physiol. 2006 Oct 15;576(Pt 2):557-67. doi: 10.1113/jphysiol.2006.111070. Epub 2006 Jul 27. J Physiol. 2006. PMID: 16873416 Free PMC article. - [Cell biology of intraocular vascular diseases].
Ishibashi T. Ishibashi T. Nippon Ganka Gakkai Zasshi. 1999 Dec;103(12):923-47. Nippon Ganka Gakkai Zasshi. 1999. PMID: 10643294 Review. Japanese. - Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase.
Ushio-Fukai M, Alexander RW. Ushio-Fukai M, et al. Mol Cell Biochem. 2004 Sep;264(1-2):85-97. doi: 10.1023/b:mcbi.0000044378.09409.b5. Mol Cell Biochem. 2004. PMID: 15544038 Review.
Cited by
- NADPH oxidase inhibitors: a decade of discovery from Nox2ds to HTS.
Cifuentes-Pagano E, Csanyi G, Pagano PJ. Cifuentes-Pagano E, et al. Cell Mol Life Sci. 2012 Jul;69(14):2315-25. doi: 10.1007/s00018-012-1009-2. Epub 2012 May 15. Cell Mol Life Sci. 2012. PMID: 22585059 Free PMC article. Review. - Antioxidants and diabetic retinopathy.
Williams M, Hogg RE, Chakravarthy U. Williams M, et al. Curr Diab Rep. 2013 Aug;13(4):481-7. doi: 10.1007/s11892-013-0384-x. Curr Diab Rep. 2013. PMID: 23649947 Review. - Vascular dysfunction in retinopathy-an emerging role for arginase.
Caldwell RB, Zhang W, Romero MJ, Caldwell RW. Caldwell RB, et al. Brain Res Bull. 2010 Feb 15;81(2-3):303-9. doi: 10.1016/j.brainresbull.2009.08.025. Epub 2009 Sep 6. Brain Res Bull. 2010. PMID: 19737603 Free PMC article. Review. - TIAM1-RAC1 signalling axis-mediated activation of NADPH oxidase-2 initiates mitochondrial damage in the development of diabetic retinopathy.
Kowluru RA, Kowluru A, Veluthakal R, Mohammad G, Syed I, Santos JM, Mishra M. Kowluru RA, et al. Diabetologia. 2014 May;57(5):1047-56. doi: 10.1007/s00125-014-3194-z. Epub 2014 Feb 20. Diabetologia. 2014. PMID: 24554007 Free PMC article. - The quest for selective nox inhibitors and therapeutics: challenges, triumphs and pitfalls.
Cifuentes-Pagano E, Meijles DN, Pagano PJ. Cifuentes-Pagano E, et al. Antioxid Redox Signal. 2014 Jun 10;20(17):2741-54. doi: 10.1089/ars.2013.5620. Epub 2013 Dec 14. Antioxid Redox Signal. 2014. PMID: 24070014 Free PMC article. Review.
References
- Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 1998;43:245–269. - PubMed
- Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, III, Klein R. Diabetic retinopathy. Diabetes Care. 1998;21:143–156. - PubMed
- Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol. 1995;113:1538–1544. - PubMed
- Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, Caldwell RW. Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev. 2003;19:442–455. - PubMed
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY004618/EY/NEI NIH HHS/United States
- EY04618/EY/NEI NIH HHS/United States
- R01 HL070215/HL/NHLBI NIH HHS/United States
- HL70215/HL/NHLBI NIH HHS/United States
- R01 EY011766/EY/NEI NIH HHS/United States
- EY11766/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical