Hepatocyte growth factor promotes lymphatic vessel formation and function - PubMed (original) (raw)
Comparative Study
. 2005 Aug 17;24(16):2885-95.
doi: 10.1038/sj.emboj.7600763. Epub 2005 Jul 28.
Affiliations
- PMID: 16052207
- PMCID: PMC1187946
- DOI: 10.1038/sj.emboj.7600763
Comparative Study
Hepatocyte growth factor promotes lymphatic vessel formation and function
Kentaro Kajiya et al. EMBO J. 2005.
Abstract
The lymphatic vascular system plays a pivotal role in mediating tissue fluid homeostasis and cancer metastasis, but the molecular mechanisms that regulate its formation and function remain poorly characterized. A comparative analysis of the gene expression of purified lymphatic endothelial cells (LEC) versus blood vascular endothelial cells (BVEC) revealed that LEC express significantly higher levels of hepatocyte growth factor receptor (HGF-R). Whereas little or no HGF-R expression was detected by lymphatic vessels of normal tissues, HGF-R was strongly expressed by regenerating lymphatic endothelium during tissue repair and by activated lymphatic vessels in inflamed skin. Treatment of cultured LEC with HGF promoted LEC proliferation, migration and tube formation. HGF-induced proliferation of LEC did not require vascular endothelial growth factor receptor-3 activation, and HGF-induced cell migration was partially mediated via integrin alpha-9. Transgenic or subcutaneous delivery of HGF promoted lymphatic vessel formation in mice, whereas systemic blockade of HGF-R inhibited lymphatic function. These results identify HGF as a novel, potent lymphangiogenesis factor, and also indicate that HGF-R might serve as a new target for inhibiting pathological lymphangiogenesis.
Figures
Figure 1
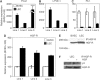
Increased expression of HGF-R by LEC. (A–C) Quantitative real-time RT–PCR confirmed that three independently established lines of primary LEC (black bars) expressed high levels of mRNA of the major lymphatic lineage markers Prox1 and LYVE-1, but expressed low levels of the blood vascular lineage marker Flt-1, as compared to BVEC (white bars). (D) LEC expressed over two-fold higher levels of HGF-R mRNA than BVEC, as determined by real-time RT–PCR. (E) Immunoprecipitation and Western blot analyses demonstrated that LEC (right lane) expressed much higher levels of HGF-R protein, compared with BVEC (left lane). (F) Treatment of LEC with 30 ng/ml HGF (right lane) resulted in increased phosphorylation of HGF-R, compared to untreated cells (left lane). IP: immunoprecipitate.
Figure 2
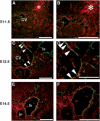
HGF-R is expressed by lymphatic vessels during inflammation and tissue repair. Double immunofluorescence stains for the lymphatic marker LYVE-1 (green) and for HGF-R (red) revealed little or no HGF-R expression by lymphatic vessels in normal mouse skin (A–C), whereas, in the inflamed skin of VEGF-A transgenic mice, indicated by LYVE-1-positive regions, enlarged lymphatic vessels (asterisks) also expressed HGF-R (D–F). HGF-R was also expressed by epidermal keratinocytes. At 21 days after induction of full-thickness wounds in mouse skin, several LYVE-1-positive, regenerating lymphatic vessels at the wound edge expressed HGF-R (G–I, inset H–L). Arrows (G–I) indicate the left border of the original wound area. Arrowheads indicate HGF-R-expressing lymphatic vessels. Scale bars: 100 μm.
Figure 3
HGF-R is expressed by endothelial cells of lymph sacs during mouse embryonic development. (A, B) By E11.5, LYVE-1-expressing endothelial cells (green) of the anterior CV expressed little or no HGF-R (red), whereas HGF-R expression was detected within the pharyngeal region of the foregut and in mesenchymal cells (asterisk). (C, D) At E12.5, weak HGF-R expression was detected in some LYVE-1-positive endothelial cells of the anterior CV (arrowheads), whereas only occasional HGF-R expression was observed near the endothelium lining the primitive lymph sacs (ls). (E, F) At E14.5, HGF-R was expressed by the vast majority of LYVE-1-positive LEC. At this stage, HGF-R was weakly expressed by endothelial cells of the jugular vein (jv) and the artery, which were LYVE-1-negative. Scale bars: 100 μm.
Figure 4
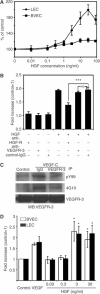
HGF promotes LEC proliferation and migration. (A) HGF induced proliferation of LEC (♦) in a dose-dependent manner, compared to untreated control cells. Proliferation was induced at a minimum effective concentration of 1 ng/ml HGF (P<0.01). HGF induced higher levels of proliferation in LEC than in BVEC (▪) at all concentrations. (B) An HGF-R-blocking antibody potently inhibited the HGF-induced stimulation of LEC proliferation (***P<0.001). Incubation with a VEGFR-3 blocking antibody or control IgG did not affect HGF-induced proliferation. (C) Treatment of LEC with VEGF-C (500 ng/ml) induced phosphorylation of VEGFR-3, which was inhibited by incubation with an anti-human VEGFR-3-neutralizing antibody. Lysates were immunoprecipitated with phosphotyrosine antibodies PY99 or 4G10 and immunoblotted with VEGFR-3. VEGFR-3 signals demonstrate the presence of equal amounts of protein. IP: Immunoprecipitate, WB: Western blot. (D) HGF induced migration of LEC and BVEC with a minimal effective concentration of 3 ng/ml. Data represent mean values ±s.e.m. of three independent experiments (*P<0.05).
Figure 5
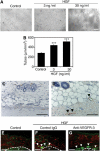
HGF promotes LEC tube formation in vitro and lymphatic vessel formation in vivo. (A, B) Treatment of LEC with 3 and 30 ng/ml HGF promoted cord formation in response to overlay with type I collagen, compared with untreated control cultures. Data are expressed as mean values ±s.d. (***P<0.001). Control Matrigels (C) and those that contain HGF (D) were subcutaneously implanted in FVB mice. Immunostaining for the lymphatic-specific glycoprotein podoplanin (red) revealed pronounced formation of new lymphatic vessels (D; arrowheads) within HGF-containing matrigels at day 7 after implantation, whereas no lymphatic vessel formation was observed within control matrigels. (E–G) Double immunofluorescence analyses of mouse ear sections for LYVE-1 (green) and CD31 (red) showed induction of LYVE-1-positive lymphatic vessel formation at 14 days after implantation of HGF-containing slow-release pellets (F and G; arrowheads), but not that of control pellets (E). Systemic treatment with a blocking anti-VEGFR-3 antibody (G) did not inhibit HGF-induced lymphatic vessel formation, as compared with control IgG treatment (F). Newly formed blood vessels were observed in all samples. P: Pellet. Scale bars: 100 μm (A, C–G).
Figure 6
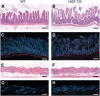
Enhanced formation of lymphatic vessels in HGF transgenic mice. Representative histologic images (H&E stain) of the ileum revealed vascular enlargement in HGF transgenic mice (B), as compared with wild-type mice (A). Immunofluorescence stains for the lymphatic vessel marker podoplanin (red) revealed enlarged central lacteals and enlarged submucosal lymphatic vessels in HGF transgenic mice (D), as compared with wild-type mice (C). Nuclei are labeled blue (Hoechst stain). (E–H) No major differences were observed in the histoarchitecture of the skin of HGF transgenic (F) and wild-type mice (E). However, podoplanin stains (red) revealed numerous enlarged lymphatic vessels in the skin of HGF transgenic mice (H) as compared with wild-type mice (G). Scale bars: 100 μm.
Figure 7
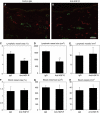
HGF-R blockade inhibits lymphatic but not blood vessel enlargement in an experimental model of skin inflammation. (A, B) Double immunofluorescence analysis for LYVE-1 (green) and CD31 (red) revealed that the characteristic enlargement of lymphatic vessels at 24 h after induction of inflammation was inhibited in mice treated with a blocking anti-HGF-R antibody (B), as compared with the control IgG-treated group (A). Scale bars: 100 μm. (C–H) Computer-assisted morphometric analyses demonstrated that the relative tissue area occupied by lymphatic vessels (C) and the average size of lymphatic vessels (D) were significantly reduced after blockade of HGF-R (**P<0.01), compared to control IgG-treated mice (C, D). No significant differences of the density of lymphatic vessels were found between both groups (E), and there were no significant differences of the area occupied by blood vessels (F), the average blood vessel size (G) and the number of blood vessels (H).
Figure 8
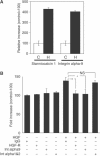
HGF promotes LEC migration via integrin alpha-9. (A) SYBR-Green-based quantitative real-time RT–PCR shows that the expression of stanniocalcin 1 and of integrin alpha-9 are significantly upregulated after treatment of LEC with HGF (H) as compared with control (C). The expression levels were normalized to those of β-actin. (B) Treatment of LEC with HGF induces cell migration, but coincubation of cells with an integrin alpha-9-specific blocking antibody prevents this migration (*P<0.05). Incubation with an HGF-R-blocking antibody also inhibited the induction of LEC migration by HGF (**P<0.01), whereas LEC migration in the absence of HGF was not affected by treatment with an integrin alpha-9-blocking antibody or an HGF-R-blocking antibody. Treatment with control IgG or combined treatment with blocking antibodies against the integrin alpha-1 and -2 had no effect on HGF-induced LEC migration. Data represent mean values ±s.e.m. (_n_=5 per group), and are representative of three independent experiments.
Similar articles
- The β1-integrin plays a key role in LEC invasion in an optimized 3-D collagen matrix model.
Kumaravel S, Abbey CA, Bayless KJ, Chakraborty S. Kumaravel S, et al. Am J Physiol Cell Physiol. 2020 Dec 1;319(6):C1045-C1058. doi: 10.1152/ajpcell.00299.2020. Epub 2020 Oct 14. Am J Physiol Cell Physiol. 2020. PMID: 33052069 - Transfection of human hepatocyte growth factor gene ameliorates secondary lymphedema via promotion of lymphangiogenesis.
Saito Y, Nakagami H, Morishita R, Takami Y, Kikuchi Y, Hayashi H, Nishikawa T, Tamai K, Azuma N, Sasajima T, Kaneda Y. Saito Y, et al. Circulation. 2006 Sep 12;114(11):1177-84. doi: 10.1161/CIRCULATIONAHA.105.602953. Epub 2006 Sep 4. Circulation. 2006. PMID: 16952986 - [Experimental study of the hepatocyte growth factor contributing to lymphangiogenesis and lymphatic metastasis in gastric cancer].
Zhang QH, Qian K, Li XJ, Pu J, Wu XT. Zhang QH, et al. Zhonghua Wei Chang Wai Ke Za Zhi. 2007 May;10(3):212-6. Zhonghua Wei Chang Wai Ke Za Zhi. 2007. PMID: 17520376 Chinese. - Methods to study lymphatic vessel integrins.
Garmy-Susini B, Makale M, Fuster M, Varner JA. Garmy-Susini B, et al. Methods Enzymol. 2007;426:415-38. doi: 10.1016/S0076-6879(07)26018-5. Methods Enzymol. 2007. PMID: 17697894 Review. - Roles of prostaglandins in tumor-associated lymphangiogenesis with special reference to breast cancer.
Lala PK, Nandi P, Majumder M. Lala PK, et al. Cancer Metastasis Rev. 2018 Sep;37(2-3):369-384. doi: 10.1007/s10555-018-9734-0. Cancer Metastasis Rev. 2018. PMID: 29858743 Review.
Cited by
- Lymphatic system regulation of anti-cancer immunity and metastasis.
Lei PJ, Fraser C, Jones D, Ubellacker JM, Padera TP. Lei PJ, et al. Front Immunol. 2024 Aug 15;15:1449291. doi: 10.3389/fimmu.2024.1449291. eCollection 2024. Front Immunol. 2024. PMID: 39211044 Free PMC article. Review. - Lymphatic endothelial cell-targeting lipid nanoparticles delivering VEGFC mRNA improve lymphatic function after injury.
Michalaki E, Chin R, Jeong K, Qi Z, Liebman LN, González-Vargas Y, Echeverri ES, Paunovska K, Muramatsu H, Pardi N, Tamburini BJ, Jakus Z, Dahlman JE, Dixon JB. Michalaki E, et al. bioRxiv [Preprint]. 2024 Jul 31:2024.07.31.605343. doi: 10.1101/2024.07.31.605343. bioRxiv. 2024. PMID: 39131391 Free PMC article. Preprint. - Molecular pathophysiology of secondary lymphedema.
Lee SO, Kim IK. Lee SO, et al. Front Cell Dev Biol. 2024 Jul 8;12:1363811. doi: 10.3389/fcell.2024.1363811. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39045461 Free PMC article. Review. - A HGF Mutation in the Familial Case of Primary Lymphedema: A Report.
Koksharova G, Kokh N, Gridina M, Khapaev R, Nimaev V, Fishman V. Koksharova G, et al. Int J Mol Sci. 2024 May 17;25(10):5464. doi: 10.3390/ijms25105464. Int J Mol Sci. 2024. PMID: 38791500 Free PMC article. - Pathogenic variants in HGF give rise to childhood-to-late onset primary lymphoedema by loss of function.
Alpaslan M, Fastré E, Mestre S, van Haeringen A, Repetto GM, Keymolen K, Boon LM, Belva F, Giacalone G, Revencu N, Sznajer Y, Riches K, Keeley V, Mansour S, Gordon K, Martin-Almedina S, Dobbins S, Ostergaard P, Quere I, Brouillard P, Vikkula M. Alpaslan M, et al. Hum Mol Genet. 2024 Jul 6;33(14):1250-1261. doi: 10.1093/hmg/ddae060. Hum Mol Genet. 2024. PMID: 38676400
References
- Alitalo K, Carmeliet P (2002) Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 1: 219–227 - PubMed
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF (2003) Met, metastasis, motility and more. Nat Rev Mol Cell Biol 4: 915–925 - PubMed
- Cao R, Bjorndahl MA, Religa P, Clasper S, Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, Ohhashi T, Jackson DG, Cao Y (2004) PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell 6: 333–345 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA86410/CA/NCI NIH HHS/United States
- R01 CA069184/CA/NCI NIH HHS/United States
- P01 CA092644/CA/NCI NIH HHS/United States
- CA92644/CA/NCI NIH HHS/United States
- CA69184/CA/NCI NIH HHS/United States
- R01 CA086410/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases