Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia - PubMed (original) (raw)
Clinical Trial
Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia
Isaac Schechter et al. Clin Neurophysiol. 2005 Sep.
Abstract
Objective: Patients with schizophrenia demonstrate significant impairments of early visual processing, potentially implicating dysfunction of the magnocellular visual pathway. The present study evaluates transient visual evoked potential (tVEP) responses to stimuli biased toward the magnocellular (M) or parvocellular (P) systems in patients with schizophrenia vs. normal volunteers first to evaluate relative contributions of M and P systems to specific tVEP components in schizophrenia and, second, to evaluate integrity of early M and P processing in schizophrenia.
Methods: Seventy-four patients with schizophrenia and schizoaffective disorder were compared with 59 control subjects using separate stimuli to assess the tVEP response to M, P and mixed M/P conditions. Stimuli were biased toward M vs. P processing by manipulation of chromatic and achromatic contrast. C1, P1, N1 and P2 components were compared between patients and controls. All subjects showed 20/32 vision or better.
Results: Waveforms were obtained to low contrast (M), chromatic contrast (P) and high contrast (mixed M/P) stimuli in both patients and controls. C1 was present to P and mixed M/P stimuli. Patients showed a significant reduction in amplitude and an increase in latency of the C1 component. P1 was elicited primarily by M and mixed M/P stimuli, whereas N1 was elicited primarily by P and mixed M/P stimuli. Patients showed reductions in both P1 and N1 amplitudes across conditions. However, only reductions in P1 amplitude survived covariation for between group differences in visual acuity. Further, P1 amplitude reductions in the M condition correlated with a proxy measure of global outcome.
Conclusions: M- and P-selective stimuli elicit differential components of the tVEP. Patients with schizophrenia show significant reductions in response even to simple visual stimuli. Deficits, particularly within the M system, may correlate significantly with global outcome and level of community functioning.
Significance: Whereas deficits in high-order cognitive processing have been extensively documented in schizophrenia, integrity of early-stage sensory processing has been studied to a lesser degree. The present findings suggest that deficits in early-stage visual processing are significantly related to overall clinical outcome in schizophrenia. Further, between-group differences in visual acuity may influence VEP results, even for subjects with 'normal' vision (20/32 or better).
Figures
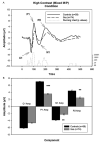
Fig. 1
A. Graph showing the group average waveforms for the high contrast (mixed M/P) condition over time for controls and patients with running _t_-test. B. Bar graph showing group average C1, P1, N1 and P2 component amplitudes with SEM for patients and controls. **P<.005, ***P<.001
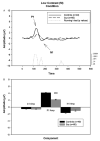
Fig. 2
A. Graph showing the group average waveforms for the low contrast (M) condition over time for controls and patients with running _t_-test. B. Bar graph showing group average C1, P1, and N1 component amplitudes with SEM for patients and controls. ***P<.001
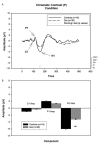
Fig. 3
A. Graph showing the group average waveforms for the chromatic contrast (P) condition over time for controls and patients with running _t_-test. B. Bar graph showing group average C1, P1, and N1 component amplitudes with SEM for patients and controls. **P<.005.
Similar articles
- Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments.
Butler PD, Martinez A, Foxe JJ, Kim D, Zemon V, Silipo G, Mahoney J, Shpaner M, Jalbrzikowski M, Javitt DC. Butler PD, et al. Brain. 2007 Feb;130(Pt 2):417-30. doi: 10.1093/brain/awl233. Epub 2006 Sep 19. Brain. 2007. PMID: 16984902 Free PMC article. - Early-stage visual processing and cortical amplification deficits in schizophrenia.
Butler PD, Zemon V, Schechter I, Saperstein AM, Hoptman MJ, Lim KO, Revheim N, Silipo G, Javitt DC. Butler PD, et al. Arch Gen Psychiatry. 2005 May;62(5):495-504. doi: 10.1001/archpsyc.62.5.495. Arch Gen Psychiatry. 2005. PMID: 15867102 Free PMC article. - Dysfunction of early-stage visual processing in schizophrenia.
Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. Butler PD, et al. Am J Psychiatry. 2001 Jul;158(7):1126-33. doi: 10.1176/appi.ajp.158.7.1126. Am J Psychiatry. 2001. PMID: 11431235 - A few observations on linking VEP responses to the magno- and parvocellular systems by way of contrast-response functions.
Skottun BC. Skottun BC. Int J Psychophysiol. 2014 Mar;91(3):147-54. doi: 10.1016/j.ijpsycho.2014.01.005. Epub 2014 Jan 15. Int J Psychophysiol. 2014. PMID: 24440598 Review. - Spatial frequency selectivity of the human visual cortex estimated with pseudo-random visual evoked cortical potential (VECP).
Martins ICVS, Brasil A, Miquilini L, Goulart PRK, Herculano AM, Silveira LCL, Souza GS. Martins ICVS, et al. Vision Res. 2019 Dec;165:13-21. doi: 10.1016/j.visres.2019.09.004. Epub 2019 Oct 11. Vision Res. 2019. PMID: 31610286 Review.
Cited by
- Increased extent of object-selective cortex in schizophrenia.
Wynn JK, Green MF, Engel S, Korb A, Lee J, Glahn D, Nuechterlein KH, Cohen MS. Wynn JK, et al. Psychiatry Res. 2008 Nov 30;164(2):97-105. doi: 10.1016/j.pscychresns.2008.01.005. Epub 2008 Oct 19. Psychiatry Res. 2008. PMID: 18938066 Free PMC article. - Differential relationships of mismatch negativity and visual p1 deficits to premorbid characteristics and functional outcome in schizophrenia.
Friedman T, Sehatpour P, Dias E, Perrin M, Javitt DC. Friedman T, et al. Biol Psychiatry. 2012 Mar 15;71(6):521-9. doi: 10.1016/j.biopsych.2011.10.037. Epub 2011 Dec 21. Biol Psychiatry. 2012. PMID: 22192361 Free PMC article. - Magnocellular pathway impairment in schizophrenia: evidence from functional magnetic resonance imaging.
Martínez A, Hillyard SA, Dias EC, Hagler DJ Jr, Butler PD, Guilfoyle DN, Jalbrzikowski M, Silipo G, Javitt DC. Martínez A, et al. J Neurosci. 2008 Jul 23;28(30):7492-500. doi: 10.1523/JNEUROSCI.1852-08.2008. J Neurosci. 2008. PMID: 18650327 Free PMC article. - Impaired conscious access and abnormal attentional amplification in schizophrenia.
Berkovitch L, Del Cul A, Maheu M, Dehaene S. Berkovitch L, et al. Neuroimage Clin. 2018 Mar 15;18:835-848. doi: 10.1016/j.nicl.2018.03.010. eCollection 2018. Neuroimage Clin. 2018. PMID: 29876269 Free PMC article. - Multiple forms of contour grouping deficits in schizophrenia: what is the role of spatial frequency?
Keane BP, Erlikhman G, Kastner S, Paterno D, Silverstein SM. Keane BP, et al. Neuropsychologia. 2014 Dec;65:221-33. doi: 10.1016/j.neuropsychologia.2014.10.031. Epub 2014 Nov 1. Neuropsychologia. 2014. PMID: 25446968 Free PMC article.
References
- Aine CJ, Supek S, George JS. Temporal dynamics of visual-evoked neuromagnetic sources: effects of stimulus parameters and selective attention. Int J Neurosci. 1995;80:79–104. - PubMed
- Allison T, Puce A, Spencer D, McCarthy G. Electrophysiological studies of human face perception I: potentials generated in occipitotemporal cortex by face and non-face stimuli. Cereb Cortex. 1999;9:415–30. - PubMed
- Andreasen NC. The scale for the assessment of negative symptoms (SANS) Iowa City, IA: The University of Iowa; 1984.
- Basinska A. Altered electrophysiological pattern of target detection in schizophrenia in the continuous attention test. Acta Neurobiol Exp. 1998;58:207–20. - PubMed
- Bentin S, Mouchetant-Rostaing Y, Giard MH, Echallier JF, Pernier J. ERP manifestations of processing printed words at different psycholinguistic levels: time course and scalp distribution. J Cognit Neurosci. 1999;11:235–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH049334/MH/NIMH NIH HHS/United States
- R01 MH66374/MH/NIMH NIH HHS/United States
- R01 MH49334/MH/NIMH NIH HHS/United States
- K02 MH001439/MH/NIMH NIH HHS/United States
- R01 MH066374/MH/NIMH NIH HHS/United States
- K02 MH01439/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical