Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function - PubMed (original) (raw)
Review
Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function
Carlos M Ferrario et al. Am J Physiol Heart Circ Physiol. 2005 Dec.
Abstract
Angiotensin-converting enzyme 2 (ACE2) is the first human homologue of ACE to be described. ACE2 is a type I integral membrane protein that functions as a carboxypeptidase, cleaving a single hydrophobic/basic residue from the COOH-terminus of its substrates. Because ACE2 efficiently hydrolyzes the potent vasoconstrictor angiotensin II to angiotensin (1-7), this has changed our overall perspective about the classical view of the renin angiotensin system in the regulation of hypertension and heart and renal function, because it represents the first example of a feedforward mechanism directed toward mitigation of the actions of angiotensin II. This paper reviews the new data regarding the biochemistry of angiotensin-(1-7)-forming enzymes and discusses key findings such as the elucidation of the regulatory mechanisms participating in the expression of ACE2 and angiotensin-(1-7) in the control of the circulation.
Figures
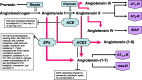
Fig. 1.
Current view of renin angiotensin system enzymes and peptides. ACE, angiotensin-converting enzyme; ACE2, angiotensin-converting enzyme 2; EPs, endopeptidases (neprilysin, prolyl endopeptidase 24.26, and metalol protease 24.15) (109) controlling tissue-specific production of angiotensin-(1–7) [ANG-(1–7)] from ANG I [ANG-(1–10)]; ANG receptors are AT1-R, AT2-R, and AT1–7-R, [type 1, type 2, and ANG-(1–7) receptor subtypes, respectively] and insulin-regulated aminopeptidase (IRAP) (84). Nomenclature for ANG peptides follows the recommendations of the Nomenclature Committee for the Council for High Blood Pressure Research (13) as the following: angiotensin II [angiotensin-(1–8)]; angiotensin III [angiotensin-(2–8)]; angiotensin IV [angiotensin-(3–8)]. Call outs denote additional enzymatic cleavage activity of the angiotensins-forming enzymes.
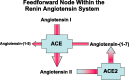
Fig. 2.
Schematic representation of feedforward enzymatic pathway through which ANG II is cleaved from ANG I by ACE and then further catalyzed by ACE2 into ANG-(1–7). In turn, ANG-(1–7) is metabolized by ACE into the ANG fragment ANG-(1–5).
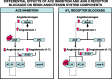
Fig. 3.
Representative outline of changes induced by ACE inhibitors (ACEI) or ANG II type 1 receptor blockers (ARBs). Other abbreviations as in Fig. 1.
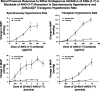
Fig. 4.
Conscious instrumented spontaneously hypertensive rats and [mRen2]27 transgenic hypertensive rats exposed to either a normal (0.5%) or a low (0.05%) -salt diet regimen for 12 days were administered systemic doses of either an affinity purified ANG-(1–7) antibody or the selective ANG-(1–7) receptor antagonist [(
d
-Ala7)-ANG-(1–7)]. Both endogenous neutralization of ANG-(1–7) and blockade of AT-1–7 receptors are associated with dose-dependent increases in the mean arterial pressure (MAP) of salt-depleted rats confirming a buffering role of the heptapeptide in which the condition of salt depletion causes increases in plasma renin activity and ANG II. Adapted from Iyer et al. (53).
Similar articles
- Angiotensin-converting enzyme-2: a molecular and cellular perspective.
Warner FJ, Smith AI, Hooper NM, Turner AJ. Warner FJ, et al. Cell Mol Life Sci. 2004 Nov;61(21):2704-13. doi: 10.1007/s00018-004-4240-7. Cell Mol Life Sci. 2004. PMID: 15549171 Free PMC article. Review. - Effects of renin-angiotensin system blockade on renal angiotensin-(1-7) forming enzymes and receptors.
Ferrario CM, Jessup J, Gallagher PE, Averill DB, Brosnihan KB, Ann Tallant E, Smith RD, Chappell MC. Ferrario CM, et al. Kidney Int. 2005 Nov;68(5):2189-96. doi: 10.1111/j.1523-1755.2005.00675.x. Kidney Int. 2005. PMID: 16221218 - A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9.
Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. Donoghue M, et al. Circ Res. 2000 Sep 1;87(5):E1-9. doi: 10.1161/01.res.87.5.e1. Circ Res. 2000. PMID: 10969042 - Regulation of ACE2 and ANG-(1-7) in the aorta: new insights into the renin-angiotensin system in the control of vascular function.
Dantas AP, Sandberg K. Dantas AP, et al. Am J Physiol Heart Circ Physiol. 2005 Sep;289(3):H980-1. doi: 10.1152/ajpheart.00476.2005. Am J Physiol Heart Circ Physiol. 2005. PMID: 16100255 Review. No abstract available. - ACE2 and vasoactive peptides: novel players in cardiovascular/renal remodeling and hypertension.
Mendoza-Torres E, Oyarzún A, Mondaca-Ruff D, Azocar A, Castro PF, Jalil JE, Chiong M, Lavandero S, Ocaranza MP. Mendoza-Torres E, et al. Ther Adv Cardiovasc Dis. 2015 Aug;9(4):217-37. doi: 10.1177/1753944715597623. Epub 2015 Aug 13. Ther Adv Cardiovasc Dis. 2015. PMID: 26275770 Review.
Cited by
- Pathomechanism and Management of Stroke in COVID-19: Review of Immunopathogenesis, Coagulopathy, Endothelial Dysfunction, and Downregulation of ACE2.
Rahmawati PL, Tini K, Susilawathi NM, Wijayanti IAS, Samatra DP. Rahmawati PL, et al. J Clin Neurol. 2021 Apr;17(2):155-163. doi: 10.3988/jcn.2021.17.2.155. J Clin Neurol. 2021. PMID: 33835735 Free PMC article. Review. - Intensive care unit-acquired weakness: A review from molecular mechanisms to its impact in COVID-2019.
Gonzalez A, Abrigo J, Achiardi O, Simon F, Cabello-Verrugio C. Gonzalez A, et al. Eur J Transl Myol. 2022 Aug 26;32(3):10511. doi: 10.4081/ejtm.2022.10511. Eur J Transl Myol. 2022. PMID: 36036350 Free PMC article. - The complex combination of COVID-19 and diabetes: pleiotropic changes in glucose metabolism.
Mahrooz A, Muscogiuri G, Buzzetti R, Maddaloni E. Mahrooz A, et al. Endocrine. 2021 May;72(2):317-325. doi: 10.1007/s12020-021-02729-7. Epub 2021 Apr 22. Endocrine. 2021. PMID: 33886062 Free PMC article. Review. - Angiotensin-(1-7) attenuates the chronotropic response to angiotensin II via stimulation of PTEN in the spontaneously hypertensive rat neurons.
Modgil A, Zhang Q, Pingili A, Singh N, Yao F, Ge J, Guo L, Xuan C, O'Rourke ST, Sun C. Modgil A, et al. Am J Physiol Heart Circ Physiol. 2012 Mar 1;302(5):H1116-22. doi: 10.1152/ajpheart.00832.2011. Epub 2011 Dec 23. Am J Physiol Heart Circ Physiol. 2012. PMID: 22198171 Free PMC article. - Is Sex a Determinant of COVID-19 Infection? Truth or Myth?
Groban L, Wang H, Sun X, Ahmad S, Ferrario CM. Groban L, et al. Curr Hypertens Rep. 2020 Aug 27;22(9):62. doi: 10.1007/s11906-020-01073-x. Curr Hypertens Rep. 2020. PMID: 32852624 Free PMC article. Review.
References
- Allred AJ, Chappell MC, Ferrario CM, and Diz DI. Differential actions of renal ischemic injury on the intrarenal angiotensin system. Am J Physiol Renal Physiol : F636–F645, 2000. - PubMed
- Allred AJ, Diz DI, Ferrario CM, and Chappell MC. Pathways for angiotensin-(1–7) metabolism in pulmonary and renal tissues. Am J Physiol Renal Physiol : F841–F850, 2000. - PubMed
- Ambuhl P, Felix D, Imboden H, Khosla MC, and Ferrario CM. Effects of angiotensin analogues and angiotensin receptor antagonists on paraventricular neurones. Regul Pept : 111–120, 1992. - PubMed
- Ardaillou R. Active fragments of angiotensin II: enzymatic pathways of synthesis and biological effects. Curr Opin Nephrol Hypertens : 28–34, 1997. - PubMed
- Averill DB, Ishiyama Y, Chappell MC, and Ferrario CM. Cardiac angiotensin-(1–7) in ischemic cardiomyopathy. Circulation : 2141–2146, 2003. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous