Loss of the serine/threonine kinase fused results in postnatal growth defects and lethality due to progressive hydrocephalus - PubMed (original) (raw)
. 2005 Aug;25(16):7054-68.
doi: 10.1128/MCB.25.16.7054-7068.2005.
Marie Evangelista, Shiuh-Ming Luoh, Gretchen D Frantz, Sreedevi Chalasani, Richard A D Carano, Marjie van Hoy, Julio Ramirez, Annie K Ogasawara, Leanne M McFarland, Ellen H Filvaroff, Dorothy M French, Frederic J de Sauvage
Affiliations
- PMID: 16055717
- PMCID: PMC1190232
- DOI: 10.1128/MCB.25.16.7054-7068.2005
Loss of the serine/threonine kinase fused results in postnatal growth defects and lethality due to progressive hydrocephalus
Mark Merchant et al. Mol Cell Biol. 2005 Aug.
Abstract
The Drosophila Fused (Fu) kinase is an integral component of the Hedgehog (Hh) pathway that helps promote Hh-dependent gene transcription. Vertebrate homologues of Fu function in the Hh pathway in vitro, suggesting that Fu is evolutionarily conserved. We have generated fused (stk36) knockout mice to address the in vivo function of the mouse Fu (mFu) homologue. fused knockouts develop normally, being born in Mendelian ratios, but fail to thrive within 2 weeks, displaying profound growth retardation with communicating hydrocephalus and early mortality. The fused gene is expressed highly in ependymal cells and the choroid plexus, tissues involved in the production and circulation of cerebral spinal fluid (CSF), suggesting that loss of mFu disrupts CSF homeostasis. Similarly, fused is highly expressed in the nasal epithelium, where fused knockouts display bilateral suppurative rhinitis. No obvious defects were observed in the development of organs where Hh signaling is required (limbs, face, bones, etc.). Specification of neuronal cell fates by Hh in the neural tube was normal in fused knockouts, and induction of Hh target genes in numerous tissues is not affected by the loss of mFu. Furthermore, stimulation of fused knockout cerebellar granule cells to proliferate with Sonic Hh revealed no defect in Hh signal transmission. These results show that the mFu homologue is not required for Hh signaling during embryonic development but is required for proper postnatal development, possibly by regulating the CSF homeostasis or ciliary function.
Figures
FIG. 1.
Generation of fused knockout mice. (A) Schematic of the fused gene targeting construct. The wild-type mouse fused locus is depicted (top), with dashed lines indicating the regions of homology contained in the targeting construct (middle) and the resulting knockout locus following proper recombination (bottom). Exons 1 and 2 are 5′ noncoding (gray), exons 3 to 9 encode the kinase domain of mFu (red), and exons 10 to 28 encode the long carboxy-terminal regulatory region (blue). Insertion of the neo cassette into the fused locus results in insertion after the initiation codon in exon 3, resulting in a nonsense transcript. Restriction enzyme sites EcoRI (E) and SacI (S) are denoted, the locations of 5′ and 3′ Southern blotting probes are depicted in blue, the location of the TaqMan primer-probe set is indicated in green, the positions of RT-PCRoligonucleotides are indicated as red arrows above and below the exons to which they anneal, and lettered arrows indicate the locations of oligonucleotides used to genotype fused knockout mice. (B) Genotyping of fused knockout (KO) mice. The upper band corresponds to a 768-bp product formed with oligonucleotides A and B from the intact fused locus, while the lower band corresponds to a 512-bp product formed with oligonucleotides C an B from the recombined fused locus. WT, wild type; Het, heterozygous. (C) Southern blot assay confirmation of homologous recombination. EcoRI digestion and Southern blotting using the 5′ probe confirmed proper recombination of the short arm, while SacI digestion and Southern blotting using the 3′ probe confirmed the proper recombination of the long arm. (D) Expression analysis of fused in wild-type (WT, blue) and fused mutant (_fu_−/−, red) p7 tissues by quantitative RT-PCR. Data are graphed as a percentage, relative to the expression levels to the ubiquitous transcript RPL19, with brain RNA samples set to 100%. Standard RT-PCR results are shown for exons 9 to 12, 19 to 25, and 27 to 28 and mouse hypoxanthine phosphoribosyltransferase amplified from p3 brain RNAs. The expected RT-PCR products are indicated by blue arrowheads. dH2O, distilled water; Exp., expected.
FIG. 2.
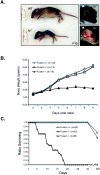
Gross observations of fused knockout mice. (A) Physical appearance of the fused knockout mouse at p10. The fused knockouts (_fu_−/−) are dramatically runty compared to wild-type (WT) littermate controls and display apparent hydrocephalus, as indicated by domed crania (right side). The skin from the neonate in the right top image was removed and photographed (lower right image) to reveal cranial swelling. (B) Body weights of wild-type (WT, open diamonds), fused heterozygous (fu+/−, gray squares), and fused knockout (_fu_−/−, black triangles) animals following birth. (C) Survival plot of wild-type (WT, open diamonds), fused heterozygous (fu+/−, gray squares), and fused knockout (_fu_−/−, black triangles) animals following birth.
FIG. 3.
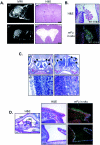
Areas of high fused expression correlate with hydrocephalus and suppurative rhinitis. (A) fused knockout mice develop a communicating form of hydrocephalus. Wild-type (WT) and fused knockout (_fu_−/−) mice are shown by MRI on the left, and brain sections stained with H&E are shown on the right. _fu_−/− mice develop a progressive, communicating form of hydrocephalus. (B) The fused mRNA is expressed highly in the choroid plexus. The top image shows H&E staining of an E16.5 mouse brain, while the bottom image shows the anti-fused radiolabeled in situexposure (white areas are positively stained with fused antisense probes). (C) _fu_−/− mice develop bilateral suppurative rhinitis characterized by massive infiltration by neutrophils. The top images show saggital sections of WT and _fu_−/− nasal cavities at a magnification of ×10, whereas the lower images show the nasal epithelial border in WT and _fu_−/− mice at a magnification of ×40. WT nasal cavities have a well-defined border at the nasal epithelium with no infiltrating cells (arrows), whereas _fu_−/− nasal cavities are filled with infiltrating neutrophils (arrows). (D) The fused mRNA is expressed in normal nasal epithelium. The leftmost image is an H&E-stained section at a magnification of ×10. The dorsum of the nose (DN), the nasal cavity (NC), and the oral cavity (OC) are indicated. The solid box corresponds to the H&E and fused radioisotopic in situ hybridization images (magnification, ×40) in the top right images, whereas the dashed box corresponds to the bottom right images.
FIG.4.
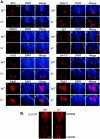
Loss of mFu does not impact Hh-dependent patterning of the ventral neural tube. (A) Specification of neural tube markers is shown for Shh (a and b), Nkx2.2 (c and d), MNR2 (e and f), Isl1 (g and h), Lim3 (i and j), Lim1/2 (k and l), Pax6 (m and n), and Pax7 (o and p). Frozen sections of E10.5 embryos were stained with monoclonal antibodies against the indicated antigens and counterstained with DAPI, and photos were taken at amagnification of ×20. No defects were observed in the specification of any neuronal population in _fu_−/− mice. (B) Specification of serotonergic neurons is normal in _fu_−/− embryos. Embryos were collected at E13.5 and stained for serotonergic specified neurons with antibodies against 5-HT. Whole-mount immunostaining of the ventral hindbrain is shown, with the rostral-most region at the top. Specification of serotonergic neurons is seen in two clusters consisting of rhombomeres 2 and 3 and 5 to 7, whereas rhombomere 4 is characteristically negative for 5-HT.
FIG. 5.
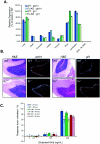
Loss of Fused does not impact Hh signal transduction in vivo or in vitro. (A) Loss of Fused does not impact Hh target gene activation in vivo. Quantitative PCR from various p7 tissues is shown for the ptch1 gene. No effects were observed on ptch1 levels in any tissue. Similar results were observed for the gli1 gene. KO, knockout; dH2O, distilled water. (B) Loss of Fused does not inhibit Hh target gene expression in the cerebellum, as measured by 32P-labeled in situ hybridization to ptch1 and gli1. Cerebellum sections (magnification, ×10) from wild-type (WT) and _fu_−/− neonates are shown stained with H&E with the comparable radiolabeled signal in the EGL for both ptch1 (left) and gli1 (right). (C) Loss of Fused does not impact the ability of cerebellar granule cells to respond to Shh. Cerebellar granule cells from wild-type, fu+/−, and _fu_−/− mice were treated in vitro for either 24 h (left image) or 48 h (right image) with octyl-modified Shh at 0, 5, 50, or 500 ng/ml. Cells were pulsed with [3H]thymidine 5 h prior to harvesting, and results are plotted as the percent response over unstimulated cells. (D) Loss of Fused does not alter ptch1 expression in vivo. High expression of ptch1 was observed in the brain (star), branchial arches (arrow), ventral CNS somites (arrowheads), and posterior limb buds of both Ptch D11 fu+/+ and Ptch D11 _fu_−/− mice. A cross section through the neural tube of these embryos shows comparable levels of ptch1 expression. (E) siRNA knockdown of mFu does not disrupt Shh-mediated signaling in C3H/10T1/2 S12 cells. Expression of mFu and mSmo was knocked down to approximately 25% and 12% of the normal levels, respectively (inset), as measured by quantitative PCR, and cells were treated with (black bars) and without (white bars) 200 ng/ml of octyl-modified Shh. No effect upon Shh-mediated activation of the 9x-Gli-BS-Luciferase reporter is observed when mFu is knocked down, while similar knockdown of mSmo totally abolishes Shh-mediated signaling. GFP, green fluorescent protein.
FIG. 5.
Loss of Fused does not impact Hh signal transduction in vivo or in vitro. (A) Loss of Fused does not impact Hh target gene activation in vivo. Quantitative PCR from various p7 tissues is shown for the ptch1 gene. No effects were observed on ptch1 levels in any tissue. Similar results were observed for the gli1 gene. KO, knockout; dH2O, distilled water. (B) Loss of Fused does not inhibit Hh target gene expression in the cerebellum, as measured by 32P-labeled in situ hybridization to ptch1 and gli1. Cerebellum sections (magnification, ×10) from wild-type (WT) and _fu_−/− neonates are shown stained with H&E with the comparable radiolabeled signal in the EGL for both ptch1 (left) and gli1 (right). (C) Loss of Fused does not impact the ability of cerebellar granule cells to respond to Shh. Cerebellar granule cells from wild-type, fu+/−, and _fu_−/− mice were treated in vitro for either 24 h (left image) or 48 h (right image) with octyl-modified Shh at 0, 5, 50, or 500 ng/ml. Cells were pulsed with [3H]thymidine 5 h prior to harvesting, and results are plotted as the percent response over unstimulated cells. (D) Loss of Fused does not alter ptch1 expression in vivo. High expression of ptch1 was observed in the brain (star), branchial arches (arrow), ventral CNS somites (arrowheads), and posterior limb buds of both Ptch D11 fu+/+ and Ptch D11 _fu_−/− mice. A cross section through the neural tube of these embryos shows comparable levels of ptch1 expression. (E) siRNA knockdown of mFu does not disrupt Shh-mediated signaling in C3H/10T1/2 S12 cells. Expression of mFu and mSmo was knocked down to approximately 25% and 12% of the normal levels, respectively (inset), as measured by quantitative PCR, and cells were treated with (black bars) and without (white bars) 200 ng/ml of octyl-modified Shh. No effect upon Shh-mediated activation of the 9x-Gli-BS-Luciferase reporter is observed when mFu is knocked down, while similar knockdown of mSmo totally abolishes Shh-mediated signaling. GFP, green fluorescent protein.
Similar articles
- Depletion of the colonic epithelial precursor cell compartment upon conditional activation of the hedgehog pathway.
van Dop WA, Uhmann A, Wijgerde M, Sleddens-Linkels E, Heijmans J, Offerhaus GJ, van den Bergh Weerman MA, Boeckxstaens GE, Hommes DW, Hardwick JC, Hahn H, van den Brink GR. van Dop WA, et al. Gastroenterology. 2009 Jun;136(7):2195-2203.e1-7. doi: 10.1053/j.gastro.2009.02.068. Epub 2009 Mar 6. Gastroenterology. 2009. PMID: 19272384 - The hedgehog signaling pathway in the mouse ovary.
Russell MC, Cowan RG, Harman RM, Walker AL, Quirk SM. Russell MC, et al. Biol Reprod. 2007 Aug;77(2):226-36. doi: 10.1095/biolreprod.106.053629. Epub 2007 Mar 28. Biol Reprod. 2007. PMID: 17392501 - Mice deficient in the fused homolog do not exhibit phenotypes indicative of perturbed hedgehog signaling during embryonic development.
Chen MH, Gao N, Kawakami T, Chuang PT. Chen MH, et al. Mol Cell Biol. 2005 Aug;25(16):7042-53. doi: 10.1128/MCB.25.16.7042-7053.2005. Mol Cell Biol. 2005. PMID: 16055716 Free PMC article. - Role and regulation of human tumor suppressor SUFU in Hedgehog signaling.
Cheng SY, Yue S. Cheng SY, et al. Adv Cancer Res. 2008;101:29-43. doi: 10.1016/S0065-230X(08)00402-8. Adv Cancer Res. 2008. PMID: 19055941 Review. - Mammalian homologues of Drosophila fused kinase.
Maloverjan A, Piirsoo M. Maloverjan A, et al. Vitam Horm. 2012;88:91-113. doi: 10.1016/B978-0-12-394622-5.00005-5. Vitam Horm. 2012. PMID: 22391301 Review.
Cited by
- Ptpn20 deletion in H-Tx rats enhances phosphorylation of the NKCC1 cotransporter in the choroid plexus: an evidence of genetic risk for hydrocephalus in an experimental study.
Xu H, Miyajima M, Nakajima M, Ogino I, Kawamura K, Akiba C, Kamohara C, Sakamoto K, Karagiozov K, Nakamura E, Tada N, Arai H, Kondo A. Xu H, et al. Fluids Barriers CNS. 2022 Jun 3;19(1):39. doi: 10.1186/s12987-022-00341-z. Fluids Barriers CNS. 2022. PMID: 35658898 Free PMC article. - Ubr3, a Novel Modulator of Hh Signaling Affects the Degradation of Costal-2 and Kif7 through Poly-ubiquitination.
Li T, Fan J, Blanco-Sánchez B, Giagtzoglou N, Lin G, Yamamoto S, Jaiswal M, Chen K, Zhang J, Wei W, Lewis MT, Groves AK, Westerfield M, Jia J, Bellen HJ. Li T, et al. PLoS Genet. 2016 May 19;12(5):e1006054. doi: 10.1371/journal.pgen.1006054. eCollection 2016 May. PLoS Genet. 2016. PMID: 27195754 Free PMC article. - Variations in Hedgehog signaling: divergence and perpetuation in Sufu regulation of Gli.
Ruel L, Thérond PP. Ruel L, et al. Genes Dev. 2009 Aug 15;23(16):1843-8. doi: 10.1101/gad.1838109. Genes Dev. 2009. PMID: 19684109 Free PMC article. - The Zn finger protein Iguana impacts Hedgehog signaling by promoting ciliogenesis.
Glazer AM, Wilkinson AW, Backer CB, Lapan SW, Gutzman JH, Cheeseman IM, Reddien PW. Glazer AM, et al. Dev Biol. 2010 Jan 1;337(1):148-56. doi: 10.1016/j.ydbio.2009.10.025. Epub 2009 Oct 21. Dev Biol. 2010. PMID: 19852954 Free PMC article. - ULK4 in Neurodevelopmental and Neuropsychiatric Disorders.
Luo S, Zheng N, Lang B. Luo S, et al. Front Cell Dev Biol. 2022 Apr 12;10:873706. doi: 10.3389/fcell.2022.873706. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35493088 Free PMC article. Review.
References
- Alcedo, J., Y. Zou, and M. Noll. 2000. Posttranscriptional regulation of smoothened is part of a self-correcting mechanism in the Hedgehog signaling system. Mol. Cell 6:457-465. - PubMed
- Alves, G., B. Limbourg-Bouchon, H. Tricoire, J. Brissard-Zahraoui, C. Lamour-Isnard, and D. Busson. 1998. Modulation of Hedgehog target gene expression by the Fused serine-threonine kinase in wing imaginal discs. Mech. Dev. 78:17-31. - PubMed
- Bose, J., L. Grotewold, and U. Ruther. 2002. Pallister-Hall syndrome phenotype in mice mutant for Gli3. Hum. Mol. Genet. 11:1129-1135. - PubMed
- Brody, S. L., X. H. Yan, M. K. Wuerffel, S. K. Song, and S. D. Shapiro. 2000. Ciliogenesis and left-right axis defects in forkhead factor HFH-4-null mice. Am. J. Respir. Cell Mol. Biol. 23:45-51. - PubMed
- Caspary, T., M. J. Garcia-Garcia, D. Huangfu, J. T. Eggenschwiler, M. R. Wyler, A. S. Rakeman, H. L. Alcorn, and K. V. Anderson. 2002. Mouse Dispatched homolog1 is required for long-range, but not juxtacrine, Hh signaling. Curr. Biol. 12:1628-1632. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases