High altitude pulmonary hypertension: role of K+ and Ca2+ channels - PubMed (original) (raw)
Review
High altitude pulmonary hypertension: role of K+ and Ca2+ channels
Carmelle V Remillard et al. High Alt Med Biol. 2005 Summer.
Abstract
Global alveolar hypoxia, as experienced at high-altitude living, has a serious impact on vascular physiology, particularly on the pulmonary vasculature. The effects of sustained hypoxia on pulmonary arteries include sustained vasoconstriction and enhanced medial hypertrophy. As the major component of the vascular media, pulmonary artery smooth muscle cells (PASMC) are the main effectors of the physiological response(s) induced during or following hypoxic exposure. Endothelial cells, on the other hand, can sense humoral and hemodynamic changes incurred by hypoxia, triggering their production of vasoactive and mitogenic factors that then alter PASMC function and growth. Transmembrane ion flux through channels in the plasma membrane not only modulates excitation- contraction coupling in PASMC, but also regulates cell volume, apoptosis, and proliferation. In this review, we examine the roles of K+ and Ca2+ channels in the pulmonary vasoconstriction and vascular remodeling observed during chronic hypoxia-induced pulmonary hypertension.
Figures
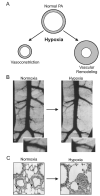
Figure. 1
Geometric and morphological changes induced by chronic hypoxia. (A) Diagram depicting hypoxia-induced vasoconstriction and vascular remodeling in pulmonary arteries (PA). Both vasoconstriction (B) and medial hypertrophy (C) are clearly demonstrated in an X-ray image (B) and lung histological preparations (C). Images in B and C are reproduced with permission from (Dawson, 2004) and (Michelakis et al., 2002), respectively.
Figure 2
Effect of sustained hypoxia on K+ channel gene transcription (A), gene expression (B), Kv currents (C), membrane potential (D), resting [Ca2+]cyt (E), and concomitant effects on both pulmonary vascular tone (F) and remodeling (G). In panels B-E inclusively, effects of hypoxia are shown in the unboxed images on the right. TF, transcription factor.
Figure 3
[K+]cyt flux influences apoptosis. Normally, the high level of cytoplasmic K+ ([K+]cyt) contributes to maintaining cell volume and suppresses (−) cytoplasmic caspase activity, thereby inhibiting the onset of apoptotic cell shrinkage and the apoptotic cascade. Opening or upregulation of K+ channels would accelerate (+) apoptotic volume decrease and enhance apoptosis by increasing K+ efflux and loss.
Figure 4
Effect of chronic hypoxia on TRPC channel expression and function in PASMC (A-B) and PAEC (C-E) in the modulation of pulmonary vascular tone and medial hypertrophy. Panels A and C describe the effects of sustained hypoxia on TRPC expression in PASMC and PAEC, respectively. Effects of hypoxia on _I_SOC and CCE are shown in panels B and D for both preparations. In PASMC, enhanced TRPC expression and CCE lead to increased PASMC contraction and proliferation. In PAEC, increased CCE enhances Ca2+-dependent AP-1 binding activity (E), thereby promoting the transcription of mitogens and growth factors such as ET-1 and PDGF, which stimulate PASMC contraction and proliferation via paracrine interactions with their respective G protein-coupled receptor (GPCR) and receptor tyrosine kinase (RTK) on PASMC membranes. Traces in panels B and D are reproduced with permission from (Lin et al., 2004) and (Fantozzi et al., 2003), respectively.
Similar articles
- Role of K+ channels in pulmonary hypertension.
Mandegar M, Yuan JX. Mandegar M, et al. Vascul Pharmacol. 2002 Jan;38(1):25-33. doi: 10.1016/s1537-1891(02)00123-4. Vascul Pharmacol. 2002. PMID: 12378819 Review. - Ion channels in pulmonary arterial hypertension.
Mandegar M, Remillard CV, Yuan JX. Mandegar M, et al. Prog Cardiovasc Dis. 2002 Sep-Oct;45(2):81-114. doi: 10.1053/pcad.2002.127491. Prog Cardiovasc Dis. 2002. PMID: 12411972 Review. - Ca(2+) and ion channels in hypoxia-mediated pulmonary hypertension.
Lai N, Lu W, Wang J. Lai N, et al. Int J Clin Exp Pathol. 2015 Feb 1;8(2):1081-92. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 25972995 Free PMC article. Review. - Molecular identification of O2 sensors and O2-sensitive potassium channels in the pulmonary circulation.
Archer SL, Weir EK, Reeve HL, Michelakis E. Archer SL, et al. Adv Exp Med Biol. 2000;475:219-40. doi: 10.1007/0-306-46825-5_21. Adv Exp Med Biol. 2000. PMID: 10849663 Review.
Cited by
- Pulmonary edema in healthy subjects in extreme conditions.
Garbella E, Catapano G, Pratali L, Pingitore A. Garbella E, et al. Pulm Med. 2011;2011:275857. doi: 10.1155/2011/275857. Epub 2011 Jun 22. Pulm Med. 2011. PMID: 21766015 Free PMC article. - Regulation of soluble guanylyl cyclase-alpha1 expression in chronic hypoxia-induced pulmonary hypertension: role of NFATc3 and HuR.
de Frutos S, Nitta CH, Caldwell E, Friedman J, González Bosc LV. de Frutos S, et al. Am J Physiol Lung Cell Mol Physiol. 2009 Sep;297(3):L475-86. doi: 10.1152/ajplung.00060.2009. Epub 2009 Jul 10. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19592461 Free PMC article. - Pulmonary vascular stiffness: measurement, modeling, and implications in normal and hypertensive pulmonary circulations.
Hunter KS, Lammers SR, Shandas R. Hunter KS, et al. Compr Physiol. 2011 Jul;1(3):1413-35. doi: 10.1002/cphy.c100005. Compr Physiol. 2011. PMID: 23733649 Free PMC article. Review. - Genomic adaptation of Ethiopian indigenous cattle to high altitude.
Terefe E, Belay G, Han J, Hanotte O, Tijjani A. Terefe E, et al. Front Genet. 2022 Dec 9;13:960234. doi: 10.3389/fgene.2022.960234. eCollection 2022. Front Genet. 2022. PMID: 36568400 Free PMC article. - High-altitude Pulmonary Hypertension: an Update on Disease Pathogenesis and Management.
Mirrakhimov AE, Strohl KP. Mirrakhimov AE, et al. Open Cardiovasc Med J. 2016 Feb 8;10:19-27. doi: 10.2174/1874192401610010019. eCollection 2016. Open Cardiovasc Med J. 2016. PMID: 27014374 Free PMC article.
References
- Abate C, Patel L, Rauscher FJ, 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990;24:1157–1161. - PubMed
- Archer SL, Huang J, Henry T, Peterson D, Weir EK. A redox-based O2 sensor in rat pulmonary vasculature. Circ. Res. 1993;73:1100–1112. - PubMed
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell. Biol. 2003;4:517–529. - PubMed
- Bonnet P, Vandier C, Cheliakine C, Garnier D. Hypoxia activates a potassium current in isolated smooth muscle cells from large pulmonary arteries of the rabbit. Exp. Physiol. 1994;79:597–600. - PubMed
- Brevnova EE, Platoshyn O, Zhang S, Yuan JX-J. Overexpression of human KCNA5 increases IK(V) and enhances apoptosis. Am. J. Physiol. Cell Physiol. 2004;287:C715–C722. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R29 HL054043/HL/NHLBI NIH HHS/United States
- U01 HL069758/HL/NHLBI NIH HHS/United States
- HL 69753/HL/NHLBI NIH HHS/United States
- R01 HL054043/HL/NHLBI NIH HHS/United States
- HL 64945/HL/NHLBI NIH HHS/United States
- HL 54043/HL/NHLBI NIH HHS/United States
- P01 HL066941/HL/NHLBI NIH HHS/United States
- HL 66941/HL/NHLBI NIH HHS/United States
- R01 HL064945/HL/NHLBI NIH HHS/United States
- HL 66012/HL/NHLBI NIH HHS/United States
- R01 HL066012/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous