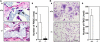DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells - PubMed (original) (raw)
. 2005 Aug 1;202(3):345-51.
doi: 10.1084/jem.20050645.
Takeshi Miyamoto, Yumi Sawatani, Katsuya Iwamoto, Naobumi Hosogane, Nobuyuki Fujita, Kozo Morita, Ken Ninomiya, Toru Suzuki, Kana Miyamoto, Yuichi Oike, Motohiro Takeya, Yoshiaki Toyama, Toshio Suda
Affiliations
- PMID: 16061724
- PMCID: PMC2213087
- DOI: 10.1084/jem.20050645
DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells
Mitsuru Yagi et al. J Exp Med. 2005.
Abstract
Osteoclasts are bone-resorbing cells that play a pivotal role in bone remodeling. Osteoclasts form large multinuclear giant cells by fusion of mononuclear osteoclasts. How cell fusion is mediated, however, is unclear. We identify the dendritic cell-specific transmembrane protein (DC-STAMP), a putative seven-transmembrane protein, by a DNA subtraction screen between multinuclear osteoclasts and mononuclear macrophages. DC-STAMP is highly expressed in osteoclasts but not in macrophages. DC-STAMP-deficient mice were generated, and osteoclast cell fusion was completely abrogated in homozygotes despite normal expression of osteoclast markers and cytoskeletal structure. As osteoclast multinucleation was restored by retroviral introduction of DC-STAMP, loss of cell fusion was directly attributable to a lack of DC-STAMP. Defects in osteoclast multinucleation reduce bone-resorbing activity, leading to osteopetrosis. Similar to osteoclasts, foreign body giant cell formation by macrophage cell fusion was also completely abrogated in DC-STAMP-deficient mice. We have thus identified an essential regulator of osteoclast and macrophage cell fusion, DC-STAMP, and an essential role of osteoclast multinucleation in bone homeostasis.
Figures
Figure 1.
Identification and gene targeting of DC-STAMP. (A) Specific expression of DC-STAMP (arrow) in osteoclasts (M-CSF + RANKL) was determined among macrophages (M-CSF alone), immature dendritic cells (M-CSF + RANKL + GM-CSF), and osteoclasts derived from common precursor cells by Northern blot analysis. (B) The endogenous DC-STAMP locus (DC-STAMP gene), targeting vector, and targeted locus (mutant allele) are shown. Exons, represented by black boxes, are numbered. The ATG start codon is located in exon 2. The neomycin resistance gene (Neo) and EGFP-polyA sequence are indicated. The probe used for Southern hybridization is indicated (P). The EGFP-polyA sequence and Neo cassette were inserted into exon 2 to yield a construct encoding a DC-STAMP (1–55)–EGFP chimeric protein. (C) RT-PCR analysis of the expression of DC-STAMP and the chimeric DC-STAMP-EGFP genes in osteoclasts and bones from mice of the indicated genotypes. (D) TRAP staining in tibias of 8-wk-old DC-STAMP+/+ or DC-STAMP−/− mice (a) and the number of multinuclear TRAP-positive cells (b). Multinuclear osteoclast formation was abrogated in DC-STAMP−/− mice. Values represent SD.
Figure 2.
Lack of multinucleation in _DC-STAMP−/−_osteoclasts. (A) TRAP expression was induced, but multinucleation was completely abrogated in DC-STAMP−/− osteoclasts. TRAP staining (a) and the number of multinuclear TRAP-positive cells (b) are shown. Values represent SD. Bar, 50 μm. (B) TRAP solution assay of macrophages (M-CSF alone) and osteoclasts (M-CSF + RANKL). (C) Expression of c-fos, NFATc1, or cathepsin K in macrophages (M-CSF alone) or osteoclasts (M-CSF + RANKL) derived from DC-STAMP+/+, DC-STAMP+/−, or DC-STAMP−/− mice was analyzed by RT-PCR. NC, no template control. (D) Ruffled border formation was detected in osteoclasts of DC-STAMP−/− tibial sections under electron microscopy. RB, ruffled border. (E) Total number of nuclei in cultured osteoclasts. Values represent SD. (F) The percent frequency of a population of osteoclast precursor cells (boxes, c-Fms+c-Kit+Mac-1low) is shown as the mean ± SD. (G) Resorbing lacunae were visualized by toluidine blue O staining (a), and relative resorbing areas were scored (b). Values represent SD.
Figure 3.
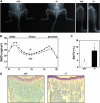
Increased bone mass in DC-STAMP−/− mice. (A) Soft x-ray analysis. Elevated radioopacity was observed in DC-STAMP−/− mice. (B) Increased BMD (mg/cm2) was detected in DC-STAMP−/− mice (closed circles) compared with DC-STAMP+/+ mice (open circles) by the dual energy x-ray absorptiometry method measured in 20 longitudinal divisions of femurs from 8-wk-old female mice. (C) Bone morphometric analysis of tibia from 8-wk-old mice. The percent BV/TV was elevated in DC-STAMP−/− mice compared with wild-type mice (*, P < 0.01). Values represent SD. (D) Increased numbers of trabeculae were observed in tibial sections of DC-STAMP−/− mice by toluidine blue staining.
Figure 4.
Rescue of cell fusion in DC-STAMP−/− osteoclasts. (A) Osteoclast precursors from DC-STAMP−/− mice were transduced by retrovirus expressing full-length (full) or a splice variant form (splice) of DC-STAMP (a). The indicated form of DC-STAMP expressed via retroviral infection was detected in _DC-STAMP−/−_osteoclasts by RT-PCR analysis (b). NC, no template control. Bar, 50 μm. (B) No multinuclear TRAP-positive cells were induced in DC-STAMP−/− cells by high density culture (a) or cocultivation with osteoblasts (b). Bar, 50 μm. (C) 2 × 104 cells/well of M-CSF–dependent osteoclast precursors from DC-STAMP−/− and DC-STAMP+/+ mice were mixed and cultured in the presence of M-CSF and RANKL for 4 d in 96-well culture plates. Cells were stained by rabbit anti-EGFP antibody followed by Alexa 488 (EGFP)–conjugated anti–rabbit IgG antibody with rhodamine-conjugated phalloidin for F-actin staining and TOTO3 for nuclear staining. Note the formation of EGFP-positive multinuclear cells. *, EGFP-negative multinuclear cells; **, EGFP-positive multinuclear cells.
Figure 5.
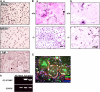
Macrophage cell fusion is abrogated in DC-STAMP−/− mice. (A) Histological analysis of implants in DC-STAMP−/− and DC-STAMP+/+ mice. Hematoxylin and eosin staining (a) and the number of FBGCs (b) are shown. Arrows indicate FBGCs. (B) FBGCs were induced and stained with May-Gruenwald Giemsa (a), and the number of FBGCs containing more than three nuclei was scored (b). Values represent SD. Bar, 50 μm.
Comment in
- Macrophage fusion: the making of osteoclasts and giant cells.
Vignery A. Vignery A. J Exp Med. 2005 Aug 1;202(3):337-40. doi: 10.1084/jem.20051123. J Exp Med. 2005. PMID: 16061722 Free PMC article. Review.
Similar articles
- Induction of DC-STAMP by alternative activation and downstream signaling mechanisms.
Yagi M, Ninomiya K, Fujita N, Suzuki T, Iwasaki R, Morita K, Hosogane N, Matsuo K, Toyama Y, Suda T, Miyamoto T. Yagi M, et al. J Bone Miner Res. 2007 Jul;22(7):992-1001. doi: 10.1359/jbmr.070401. J Bone Miner Res. 2007. PMID: 17402846 - The dendritic cell-specific transmembrane protein DC-STAMP is essential for osteoclast fusion and osteoclast bone-resorbing activity.
Miyamoto T. Miyamoto T. Mod Rheumatol. 2006;16(6):341-2. doi: 10.1007/s10165-006-0524-0. Epub 2006 Dec 20. Mod Rheumatol. 2006. PMID: 17164993 Review. - Osteoclast stimulatory transmembrane protein and dendritic cell–specific transmembrane protein cooperatively modulate cell–cell fusion to form osteoclasts and foreign body giant cells.
Miyamoto H, Suzuki T, Miyauchi Y, Iwasaki R, Kobayashi T, Sato Y, Miyamoto K, Hoshi H, Hashimoto K, Yoshida S, Hao W, Mori T, Kanagawa H, Katsuyama E, Fujie A, Morioka H, Matsumoto M, Chiba K, Takeya M, Toyama Y, Miyamoto T. Miyamoto H, et al. J Bone Miner Res. 2012 Jun;27(6):1289-97. doi: 10.1002/jbmr.1575. J Bone Miner Res. 2012. PMID: 22337159 - GM-CSF regulates fusion of mononuclear osteoclasts into bone-resorbing osteoclasts by activating the Ras/ERK pathway.
Lee MS, Kim HS, Yeon JT, Choi SW, Chun CH, Kwak HB, Oh J. Lee MS, et al. J Immunol. 2009 Sep 1;183(5):3390-9. doi: 10.4049/jimmunol.0804314. Epub 2009 Jul 29. J Immunol. 2009. PMID: 19641137 - Regulators of osteoclast differentiation and cell-cell fusion.
Miyamoto T. Miyamoto T. Keio J Med. 2011;60(4):101-5. doi: 10.2302/kjm.60.101. Keio J Med. 2011. PMID: 22200633 Review.
Cited by
- Resolvin E1 regulates osteoclast fusion via DC-STAMP and NFATc1.
Zhu M, Van Dyke TE, Gyurko R. Zhu M, et al. FASEB J. 2013 Aug;27(8):3344-53. doi: 10.1096/fj.12-220228. Epub 2013 Apr 29. FASEB J. 2013. PMID: 23629863 Free PMC article. - Update on the pathogenesis and genetics of Paget's disease of bone.
Gennari L, Rendina D, Merlotti D, Cavati G, Mingiano C, Cosso R, Materozzi M, Pirrotta F, Abate V, Calabrese M, Falchetti A. Gennari L, et al. Front Cell Dev Biol. 2022 Aug 12;10:932065. doi: 10.3389/fcell.2022.932065. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36035996 Free PMC article. Review. - Osteoclasts-Key Players in Skeletal Health and Disease.
Novack DV, Mbalaviele G. Novack DV, et al. Microbiol Spectr. 2016 Jun;4(3):10.1128/microbiolspec.MCHD-0011-2015. doi: 10.1128/microbiolspec.MCHD-0011-2015. Microbiol Spectr. 2016. PMID: 27337470 Free PMC article. Review. - Inositol hexakisphosphate inhibits osteoclastogenesis on RAW 264.7 cells and human primary osteoclasts.
Arriero Mdel M, Ramis JM, Perelló J, Monjo M. Arriero Mdel M, et al. PLoS One. 2012;7(8):e43187. doi: 10.1371/journal.pone.0043187. Epub 2012 Aug 14. PLoS One. 2012. PMID: 22905230 Free PMC article. - Osteoclast Fusion: Time-Lapse Reveals Involvement of CD47 and Syncytin-1 at Different Stages of Nuclearity.
Møller AM, Delaissé JM, Søe K. Møller AM, et al. J Cell Physiol. 2017 Jun;232(6):1396-1403. doi: 10.1002/jcp.25633. Epub 2016 Oct 19. J Cell Physiol. 2017. PMID: 27714815 Free PMC article.
References
- Grigoriadis, A.E., Z.Q. Wang, M.G. Cecchini, W. Hofstetter, R. Felix, H.A. Fleisch, and E.F. Wagner. 1994. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 266:443–448. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials