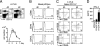Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs - PubMed (original) (raw)
Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs
Hiroyuki Yoneyama et al. J Exp Med. 2005.
Abstract
Antiviral cell-mediated immunity is initiated by the dendritic cell (DC) network in lymph nodes (LNs). Plasmacytoid DCs (pDCs) are known to migrate to inflamed LNs and produce interferon (IFN)-alpha, but their other roles in antiviral T cell immunity are unclear. We report that LN-recruited pDCs are activated to create local immune fields that generate antiviral cytotoxic T lymphocytes (CTLs) in association with LNDCs, in a model of cutaneous herpes simplex virus (HSV) infection. Although pDCs alone failed to induce CTLs, in vivo depletion of pDCs impaired CTL-mediated virus eradication. LNDCs from pDC-depleted mice showed impaired cluster formation with T cells and antigen presentation to prime CTLs. Transferring circulating pDC precursors from wild-type, but not CXCR3-deficient, mice to pDC-depleted mice restored CTL induction by impaired LNDCs. In vitro co-culture experiments revealed that pDCs provided help signals that recovered impaired LNDCs in a CD2- and CD40L-dependent manner. pDC-derived IFN-alpha further stimulated the recovered LNDCs to induce CTLs. Therefore, the help provided by pDCs for LNDCs in primary immune responses seems to be pivotal to optimally inducing anti-HSV CTLs.
Figures
Figure 1.
Recruitment and activation of pDCs in inflamed LNs. (A) The frequency (top) and the absolute numbers (bottom) of B220+CD11c+ pDCs in PLNs (rt. PLN) from uninfected (d0) or HSV-infected mice on day 2 (d2). (B) Expression of CD86, CD40, and CD40L on blood pDC precursors from uninfected (d0) or HSV-infected mice on day 2 (d2). (C) Expression of CD86, CD40, and CD40L on LN CD11c+ DCs from uninfected (d0) or HSV-infected mice on day 2 (d2). The percentages of cells are indicated. (D) IFN-α production by sorted pDC precursors from HSV-infected mice on day 2 (pDCpre) and PLN pDCs from uninfected (LN pDC d0) or HSV-infected mice on day 2 (LN pDC d2) after 16 h of incubation with irradiated HSV. (A and D) Representative values from three independent experiments are presented as the mean ± SD (n = 6).
Figure 2.
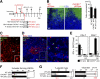
Recruited pDCs contact LNDCs in a CXCL10- and CD2-dependent manner. (A and B) Distribution of B220−CD11c+ LNDCs (red) and B220+CD11c+ pDCs (yellow) in the T cell zones in normal (A, d0) and HSV-infected (B, d2) PLN. Arrows indicate contact between pDCs and LNDCs on day 2. Bars: (A and B, left) 40 μm; (B, right) 20 μm. (C) CFSE-labeled pDC precursors (green) were adoptively transferred into HSV-infected mice on day 2. PLNs 2 h after transfer (d2) are shown. CD11c is red and B220 is blue. Transferred pDCs (green/white) are in contact with B220−CD11c+ LNDCs (bottom right, red) and B220+CD11c+ pDCs (top right, pink). (D) PLNs 48 h after transfer (d4). CCL21 is red and B220 is blue. F, follicle; PC, paracortex. Bar, (C and D) 80 μm. (E) Relative expression levels of CXCL10 by freshly isolated pDC precursors from HSV-infected mice on day 2 (pDCpre) and PLN pDCs of uninfected (LN pDC d0) or HSV-infected mice on day 2 (LN pDC d2). The housekeeping enzyme hypoxanthine phosphoribosyl transferase (HPRT) was used as an internal standard. (F) Expression of CD2 on blood pDC precursors and PLN pDCs from uninfected (d0) or HSV-infected mice on day 2 (d2). (G) CD40L expression (red) on d2 LNDC–pDC conjugates. A CFSEhigh LNDC (asterisk) and three CFSElow pDCs are shown. High-contrast image shows that CD40L is expressed on the edge of pDCs (bottom right). Bar, 20 μm. (H) The effect of blocking Abs against CD2 and CD2 + CXCL10 on the percentage of LNDCs forming clusters, using PLN DCs from uninfected (d0) and HSV-infected mice on day 2 (d2). Representative data from three independent experiments are presented as the mean ± SD (n = 6). *, P < 0.05 by the Student's t test, comparing mice treated with control and blocking Abs.
Figure 3.
In vivo depletion of pDCs impairs CTL-mediated virus eradication. The effect of anti-CXCL9 and anti–E-selectin Abs on (A) the numbers of B220− LNDCs (mDCs and CD8α+ DCs) and B220+ pDCs in PLNs after HSV infection, (B) the numbers of IFN-γ+ spots produced by PLN CD8+ T cells, (C) the specific lysis in vitro by PLN CD8+ T cells, and (D) the virus titer in PLNs. The effect of anti-Ly6G/C Ab on (E) the numbers of B220− LNDCs (mDCs and CD8α+ DCs) and B220+ pDCs in PLNs after HSV infection, (F) the numbers of IFN-γ+ spots produced by PLN CD8+ T cells, (G) the specific lysis in vitro by PLN CD8+ T cells, and (H) the virus titer in PLNs. The effect of anti–PDCA-1 mAb on (I) the numbers of B220− LNDCs (mDCs and CD8α+ DCs) and B220+ pDCs in PLNs after HSV infection, (J) the numbers of IFN-γ+ spots produced by PLN CD8+ T cells, and (K) the specific lysis in vitro by PLN CD8+ T cells. Representative data from three independent experiments are presented as the mean ± SD (n = 6 or n = 5 for anti–PDCA-1 mAb experiments. *, P < 0.05 by Student's t test, comparing mice treated with control and blocking Abs.
Figure 4.
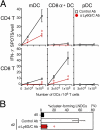
Impaired APC function of LNDCs in pDC-depleted mice. (A) The number of IFN-γ+ spots produced by CD4+ or CD8+ T cells obtained from control PLNs on day 2, after 16-h incubations with the indicated numbers of DCs with no in vitro restimulation. DCs alone formed no spots. (B) The percentage of cluster-forming LNDCs from uninfected mice (d0), HSV-infected, control Ab–treated mice on day 2, and HSV-infected, anti-Ly6G/C Ab–treated mice on day 2. Indicated LNDCs were incubated for 2 h with LN CD3+ T cells from HSV-infected mice on day 2. T cells alone rarely formed clusters (<5%) in all groups tested. Representative data from three independent experiments are presented as the mean ± SD (n = 3). *, P < 0.05 by Student's t test, comparing mice treated with control or anti-Ly6C/G Ab.
Figure 5.
Reconstitution of pDC-depleted mice with pDC precursors restores CTL induction. (A) Donor precursor cells isolated from the peripheral blood of HSV-infected WT mice (WT pDC), HSV-infected CXCR3-deficient mice (CXCR3−/− pDC), and _P. acnes_–primed WT mice (WT pDC [p.a.]) on day 2 were labeled with fluorescence dyes and adoptively transferred into HSV-infected, anti-Ly6G/C Ab–treated mice. LNDCs were isolated from reconstituted mice on day 2 of infection, and LN CD8+ T cells were isolated from reconstituted mice on day 7 of infection, for functional tests. The letters in parentheses correspond to panels in the figure. (B) Effective recruitment of WT, but not CXCR3−/−, pDC precursors on day 2 of infection. (left and middle) Entry of CMTMR-labeled pDC precursors (orange) in the T cell zone (blue, CD3; green, B220) of PLN 2 h after cell transfer. Magnification, 200. (right) The number of transferred pDCs observed in the T cell zone of PLN on day 2 of infection. Representative data from three independent experiments are presented as the mean ± SD (n = 3 for 15-mm2 sections). *, P < 0.05 by the Student's t test, comparing WT with CXCR3−/− pDCs. (C) CFSE-labeled WT pDC precursors (green) in contact with DEC-205+ (red) LNDCs in the T cell zone of PLN 2 h after cell transfer (day 2 of infection) at a magnification of 200. The inset shows a higher magnification (400). (D) CFSE-labeled WT pDC precursors in contact with BrdU+ (red) and CD4+ (blue) T cells of PLN 48 h after cell transfer (day 4 of infection) at a magnification of 200. A higher magnification is indicated by the box (400). F, follicle; PC, paracortex; S, sinus. (E) The number of IFN-γ+ spots produced by PLN CD4+ or CD8+ T cells (106 cells/well) from HSV-infected mice on day 2, after 16 h of incubation with LNDCs with no in vitro restimulation. LNDCs (mDCs or CD8α+ DCs; 105 cells/well) were obtained from anti-Ly6G/C mAb–treated mice reconstituted with WT pDC or CXCR3−/− pDCs on day 2. DCs alone produced no spots. (F) The percentage of cluster-forming LNDCs obtained from anti-Ly6G/C mAb–treated mice reconstituted with WT pDC, CXCR3−/− pDC, or WT pDC (p.a.) on day 2. Freshly isolated LNDCs were incubated with LN CD3+ T cells from HSV-infected mice for 2 h. (G) Specific lysis in vitro of PLN CD8+ T cells and virus titers of PLNs obtained from pDC-depleted mice reconstituted with WT pDCs, CXCR3−/− pDCs, or WT pDCs (p.a.) on day 7. Values for mice treated with control Ab (black dashed lines) and anti-Ly6G/C Ab (red dashed lines) on days 2 (F) and 7 (G) of infection are also shown to compare reconstituted mice with nonreconstituted mice as described in Figs. 3, G and H and Fig. 4 B. Representative data from three independent experiments are presented as the mean ± SD (n = 3). *, P < 0.05 by Student's t test, comparing WT with CXCR3−/− pDCs.
Figure 6.
CTL induction was restored using in vitro co-cultures with LN pDCs. (A) Diagram showing the protocol for co-culture experiments. CTL activities were estimated after 48 h of co-culture. The letters in parentheses correspond to panels in the figure. Ctl LN, HSV-infected PLN treated with control Ab at day 2. (B) In vitro CTL activity induced by pDCs (purple line), LNDCs (pink line), and impaired LNDCs in the presence (orange line) or absence (green line) of rIFN-α. (C) In vitro CTL activities induced by impaired LNDCs in the presence of pDCs, at ratios between 1:1 and 1:10, or with pDCs separated by transwells (red line, 1:1 ratio). (D, top) Diagram showing the protocol for two-step co-culture experiments. (top row) Step one, co-culture of impaired LNDCs and pDCs for 16 h. (bottom row) Addition of LN CD8+ T cells for a further 48 h. (bottom) In vitro CTL activities induced by impaired LNDCs in the presence of equal numbers of pDCs, treated as indicated in the figure. Effector/target (E:T) ratio, 30:1. Representative data from three independent experiments are presented as the mean ± SD (n = 3). *, P < 0.05 by Student's t test, comparing cells treated with control and blocking Abs. (E) in vitro CTL activities induced by impaired LNDCs in the presence of equal numbers of WT or CD40L−/− pDCs. E:T ratio, 30:1. Representative data from three independent experiments are presented as the mean ± SD (n = 3).
Similar articles
- Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules.
Yoneyama H, Matsuno K, Zhang Y, Nishiwaki T, Kitabatake M, Ueha S, Narumi S, Morikawa S, Ezaki T, Lu B, Gerard C, Ishikawa S, Matsushima K. Yoneyama H, et al. Int Immunol. 2004 Jul;16(7):915-28. doi: 10.1093/intimm/dxh093. Epub 2004 May 24. Int Immunol. 2004. PMID: 15159375 - Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways.
Hochrein H, Schlatter B, O'Keeffe M, Wagner C, Schmitz F, Schiemann M, Bauer S, Suter M, Wagner H. Hochrein H, et al. Proc Natl Acad Sci U S A. 2004 Aug 3;101(31):11416-21. doi: 10.1073/pnas.0403555101. Epub 2004 Jul 22. Proc Natl Acad Sci U S A. 2004. PMID: 15272082 Free PMC article. - Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming.
Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Allan RS, et al. Immunity. 2006 Jul;25(1):153-62. doi: 10.1016/j.immuni.2006.04.017. Immunity. 2006. PMID: 16860764 - Migration of dendritic cells.
Yoneyama H, Matsuno K, Matsushimaa K. Yoneyama H, et al. Int J Hematol. 2005 Apr;81(3):204-7. doi: 10.1532/IJH97.04164. Int J Hematol. 2005. PMID: 15814331 Review. - Plasmacytoid dendritic cells: in search of their niche in immune responses.
Barchet W, Blasius A, Cella M, Colonna M. Barchet W, et al. Immunol Res. 2005;32(1-3):75-83. doi: 10.1385/IR:32:1-3:075. Immunol Res. 2005. PMID: 16106060 Review.
Cited by
- DC-Based Vaccines for Cancer Immunotherapy.
Fu C, Zhou L, Mi QS, Jiang A. Fu C, et al. Vaccines (Basel). 2020 Nov 26;8(4):706. doi: 10.3390/vaccines8040706. Vaccines (Basel). 2020. PMID: 33255895 Free PMC article. Review. - Dissecting the role of dendritic cells in simian immunodeficiency virus infection and AIDS.
Wonderlich ER, Kader M, Wijewardana V, Barratt-Boyes SM. Wonderlich ER, et al. Immunol Res. 2011 Aug;50(2-3):228-34. doi: 10.1007/s12026-011-8220-3. Immunol Res. 2011. PMID: 21717075 Free PMC article. Review. - Plasmacytoid dendritic cell depletion leads to an enhanced mononuclear phagocyte response in lungs of mice with lethal influenza virus infection.
Soloff AC, Weirback HK, Ross TM, Barratt-Boyes SM. Soloff AC, et al. Comp Immunol Microbiol Infect Dis. 2012 Jul;35(4):309-17. doi: 10.1016/j.cimid.2012.01.012. Epub 2012 Mar 14. Comp Immunol Microbiol Infect Dis. 2012. PMID: 22421538 Free PMC article. - Crosstalk between human DC subsets promotes antibacterial activity and CD8+ T-cell stimulation in response to bacille Calmette-Guérin.
Lozza L, Farinacci M, Faé K, Bechtle M, Stäber M, Dorhoi A, Bauer M, Ganoza C, Weber S, Kaufmann SH. Lozza L, et al. Eur J Immunol. 2014 Jan;44(1):80-92. doi: 10.1002/eji.201343797. Epub 2013 Oct 20. Eur J Immunol. 2014. PMID: 24114554 Free PMC article. - TLR7/9 versus TLR3/MDA5 signaling during virus infections and diabetes.
Swiecki M, McCartney SA, Wang Y, Colonna M. Swiecki M, et al. J Leukoc Biol. 2011 Oct;90(4):691-701. doi: 10.1189/jlb.0311166. Epub 2011 Aug 15. J Leukoc Biol. 2011. PMID: 21844166 Free PMC article. Review.
References
- Vossen, M.T., E.M. Westerhout, C. Soderberg-Naucler, and E.J. Wiertz. 2002. Viral immune evasion: a masterpiece of evolution. Immunogenetics. 54:527–542. - PubMed
- Carbone, F.R., and W.R. Heath. 2003. The role of dendritic cell subsets in immunity to viruses. Curr. Opin. Immunol. 15:416–420. - PubMed
- Shortman, K., and Y.J. Liu. 2002. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2:151–161. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical