Hide and run. Arginine-based endoplasmic-reticulum-sorting motifs in the assembly of heteromultimeric membrane proteins - PubMed (original) (raw)
Review
Hide and run. Arginine-based endoplasmic-reticulum-sorting motifs in the assembly of heteromultimeric membrane proteins
Kai Michelsen et al. EMBO Rep. 2005 Aug.
Abstract
Arginine-based endoplasmic reticulum (ER)-localization signals are sorting motifs that are involved in the biosynthetic transport of multimeric membrane proteins. After their discovery in the invariant chain of the major histocompatibility complex class II, several hallmarks of these signals have emerged. They occur in polytopic membrane proteins that are subunits of membrane protein complexes; the presence of the signal maintains improperly assembled subunits in the ER by retention or retrieval until it is masked as a result of heteromultimeric assembly. A distinct consensus sequence and their position independence with respect to the distal termini of the protein distinguish them from other ER-sorting motifs. Recognition by the coatomer (COPI) vesicle coat explains ER retrieval. Often, di-leucine endocytic signals occur close to arginine-based signals. Recruitment of 14-3-3 family or PDZ-domain proteins can counteract ER-localization activity, as can phosphorylation. This, and the occurrence of arginine-based signals in alternatively spliced regions, implicates them in the regulated surface expression of multimeric membrane proteins in addition to their function in quality control.
Figures
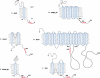
Figure 1
Arginine-based signals in proteins of different topology. Numbers indicate the size of cytosolic domains and the first Arg residue of the Arg-based signal. Arg-based signals are indicated in red. (A) Invariant chain Ii p35 of major histocompatibility complex (MHC) class II (p35 is one of two variants owing to the use of alternative initiator methionines). (B) γ-aminobutyric acid (GABAB) receptor subunit 1. The zigzag line indicates a coiled-coil forming domain. (C) Kir6.2 is the pore-forming subunit of the KATP channel. (D) SUR1 is the regulatory subunit of the KATP channel. (E) Glycoprotein B from human cytomegalovirus (HCMV). (F) N-methyl
D
-aspartate (NMDA) receptor subunit NR1-1.
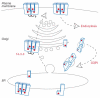
Figure 2
Model of KATP channel transport. Larger and smaller rectangular shapes represent the SUR1 and Kir6.2 subunits, respectively. Arginine (Arg)-based signals are shown as red dots. 14-3-3 proteins are depicted as joined brackets. These proteins have been implicated in the forward transport of KATP channels (Yuan et al, 2003), but their exact role is unclear (Nufer & Hauri, 2003). A di-leucine endocytosis signal in the vicinity of the Arg-based signal has been shown to mediate dynamin-dependent endocytosis (Hu et al, 2003). COPI, coat protein complex I; ER, endoplasmic reticulum.
Figure 3
Mechanisms for the masking of arginine-based signals. Red dots symbolize the Arg-based signals. (A) Steric masking: coiled-coil forming domains in the two γ-aminobutyric acid (GABAB) receptor subunits hide the signal present in GABAB R1-subunit (Margeta-Mitrovic et al, 2000). (B) Phosphorylation of a residue in the vicinity of the Arg-based signal in the major histocompatibility complex (MHC) invariant chain recruits 14-3-3 proteins (joined brackets), which leads to signal shielding (Kuwana et al, 1998). Heteromultimeric assembly with MHC class II βsubunit also leads to steric masking (Khalil et al, 2003). How the two mechanisms relate to each other is unclear. (C) Masking by a PDZ-domain protein (large bracket) binding to the distal C-terminus of N-methyl
D
-aspartate receptor subunit NR1-3 (Standley et al, 2000).
Kai Michelsen, Blanche Schwappach and Hebao Yuan. B.S. is the recipient of an EMBO Young Investigator award.
Similar articles
- 14-3-3 dimers probe the assembly status of multimeric membrane proteins.
Yuan H, Michelsen K, Schwappach B. Yuan H, et al. Curr Biol. 2003 Apr 15;13(8):638-46. doi: 10.1016/s0960-9822(03)00208-2. Curr Biol. 2003. PMID: 12699619 - Scavenging of 14-3-3 proteins reveals their involvement in the cell-surface transport of ATP-sensitive K+ channels.
Heusser K, Yuan H, Neagoe I, Tarasov AI, Ashcroft FM, Schwappach B. Heusser K, et al. J Cell Sci. 2006 Oct 15;119(Pt 20):4353-63. doi: 10.1242/jcs.03196. J Cell Sci. 2006. PMID: 17038548 - A multimeric membrane protein reveals 14-3-3 isoform specificity in forward transport in yeast.
Michelsen K, Mrowiec T, Duderstadt KE, Frey S, Minor DL, Mayer MP, Schwappach B. Michelsen K, et al. Traffic. 2006 Jul;7(7):903-16. doi: 10.1111/j.1600-0854.2006.00430.x. Epub 2006 May 25. Traffic. 2006. PMID: 16734667 - 14-3-3 proteins in membrane protein transport.
Mrowiec T, Schwappach B. Mrowiec T, et al. Biol Chem. 2006 Sep;387(9):1227-36. doi: 10.1515/BC.2006.152. Biol Chem. 2006. PMID: 16972791 Review. - Signal-mediated sorting of membrane proteins between the endoplasmic reticulum and the golgi apparatus.
Teasdale RD, Jackson MR. Teasdale RD, et al. Annu Rev Cell Dev Biol. 1996;12:27-54. doi: 10.1146/annurev.cellbio.12.1.27. Annu Rev Cell Dev Biol. 1996. PMID: 8970721 Review.
Cited by
- Calmodulin is a functional regulator of Cav1.4 L-type Ca2+ channels.
Griessmeier K, Cuny H, Rötzer K, Griesbeck O, Harz H, Biel M, Wahl-Schott C. Griessmeier K, et al. J Biol Chem. 2009 Oct 23;284(43):29809-16. doi: 10.1074/jbc.M109.048082. Epub 2009 Aug 28. J Biol Chem. 2009. PMID: 19717559 Free PMC article. - SARS-CoV-2 accessory protein ORF8 is secreted extracellularly as a glycoprotein homodimer.
Matsuoka K, Imahashi N, Ohno M, Ode H, Nakata Y, Kubota M, Sugimoto A, Imahashi M, Yokomaku Y, Iwatani Y. Matsuoka K, et al. J Biol Chem. 2022 Mar;298(3):101724. doi: 10.1016/j.jbc.2022.101724. Epub 2022 Feb 11. J Biol Chem. 2022. PMID: 35157849 Free PMC article. - Mutations in the main cytoplasmic loop of the GABA(A) receptor α4 and δ subunits have opposite effects on surface expression.
Bracamontes JR, Li P, Akk G, Steinbach JH. Bracamontes JR, et al. Mol Pharmacol. 2014 Jul;86(1):20-7. doi: 10.1124/mol.114.092791. Epub 2014 Apr 10. Mol Pharmacol. 2014. PMID: 24723490 Free PMC article. - Bidirectional modulation of thermal and chemical sensitivity of TRPM8 channels by the initial region of the N-terminal domain.
Pertusa M, González A, Hardy P, Madrid R, Viana F. Pertusa M, et al. J Biol Chem. 2014 Aug 8;289(32):21828-43. doi: 10.1074/jbc.M114.565994. Epub 2014 Jun 10. J Biol Chem. 2014. PMID: 24917670 Free PMC article. - Conserved but not critical: Trafficking and function of NaV1.7 are independent of highly conserved polybasic motifs.
Tyagi S, Sarveswaran N, Higerd-Rusli GP, Liu S, Dib-Hajj FB, Waxman SG, Dib-Hajj SD. Tyagi S, et al. Front Mol Neurosci. 2023 Mar 16;16:1161028. doi: 10.3389/fnmol.2023.1161028. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37008789 Free PMC article.
References
- Bakke O, Dobberstein B (1990) MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell 63: 707–716 - PubMed
- Chan WY, Soloviev MM, Ciruela F, McIlhinney RA (2001) Molecular determinants of metabotropic glutamate receptor 1B trafficking. Mol Cell Neurosci 17: 577–588 - PubMed
- Chang XB, Cui L, Hou YX, Jensen TJ, Aleksandrov AA, Mengos A, Riordan JR (1999) Removal of multiple arginine-framed trafficking signals overcomes misprocessing of ΔF508 CFTR present in most patients with cystic fibrosis. Mol Cell 4: 137–142 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources