Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure - PubMed (original) (raw)
Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure
Ichiro Shiojima et al. J Clin Invest. 2005 Aug.
Abstract
Although increased external load initially induces cardiac hypertrophy with preserved contractility, sustained overload eventually leads to heart failure through poorly understood mechanisms. Here we describe a conditional transgenic system in mice characterized by the sequential development of adaptive cardiac hypertrophy with preserved contractility in the acute phase and dilated cardiomyopathy in the chronic phase following the induction of an activated Akt1 gene in the heart. Coronary angiogenesis was enhanced during the acute phase of adaptive cardiac growth but reduced as hearts underwent pathological remodeling. Enhanced angiogenesis in the acute phase was associated with mammalian target of rapamycin-dependent induction of myocardial VEGF and angiopoietin-2 expression. Inhibition of angiogenesis by a decoy VEGF receptor in the acute phase led to decreased capillary density, contractile dysfunction, and impaired cardiac growth. Thus, both heart size and cardiac function are angiogenesis dependent, and disruption of coordinated tissue growth and angiogenesis in the heart contributes to the progression from adaptive cardiac hypertrophy to heart failure.
Figures
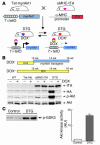
Figure 1
Generation of cardiac-specific inducible Akt1–Tg mice. (A) Schematic illustration of binary Tg system. (B) DOX-dependent expression of Akt1 transgene. Top: Temporal profile of DOX treatment. Bottom: Western blot analysis of transgene expression. (C) Kinase assay in vitro. Left: Western blot analysis of Akt substrate. GSK3, glycogen synthase kinase 3. Right: Densitometric analysis of Akt activity.
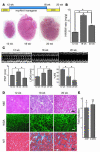
Figure 2
Cardiac hypertrophy is induced by short-term Akt activation. (A) Top: Temporal profile of DOX treatment. Bottom: Representative gross morphology of the DTG hearts. (B) HW/BW ratio. *P < 0.01. (C) Time course of cardiac hypertrophy. Top: Time course of transgene expression. Bottom: Time course of HW/BW ratio. †P < 0.01 versus day 0; #P < 0.05 versus day 14. (D) Echocardiography. Top: Representative M-mode recordings. Bottom: Posterior wall thickness (PWT), LV end-diastolic dimension (LVDd), and percent fractional shortening (%FS). **P < 0.05. (E) Histological analysis. H&E, wheat germ agglutinin (WGA), and Masson’s trichrome (MT) staining of heart sections. Scale bars: 50 μm. (F) Fold induction of ANP and β-MHC expression after short-term Akt activation.
Figure 3
Extensive hypertrophy and contractile dysfunction induced by prolonged Akt activation. (A) Top: Temporal profile of DOX treatment. Bottom: Representative gross morphology. (B) HW/BW ratio. *P < 0.01. (C) Echocardiography. Top: Representative M-mode recordings. Bottom: posterior wall thickness, LV end-diastolic dimension, and percent fractional shortening. *P < 0.01; #P < 0.05. (D) Histology: H&E, wheat germ agglutinin, and Masson’s trichrome staining of heart sections. Scale bar: 50 μm. (E) Fold induction of ANP and β-MHC expression after prolonged Akt activation. **P < 0.01 versus control; †P < 0.05 versus control.
Figure 4
Prevention of cardiac growth and heart failure progression by rapamycin. (A) HW/BW ratio of mice in the acute (left) and chronic (right) phase after Akt transgene induction. *P < 0.01. (B) Western blot analysis of Akt transgene and S6K phosphorylation in the acute (left) and chronic (right) phase after Akt transgene induction. (C) Echocardiography. Top: Representative M-mode recordings. Bottom: posterior wall thickness, LV end-diastolic dimension, and percent fractional shortening 6 weeks after transgene induction. *P < 0.01. (D) Histological analysis. H&E, wheat germ agglutinin, and Masson’s trichrome staining of heart sections 6 weeks after transgene induction. Scale bar: 50 μm. (E) Fold induction of ANP and β-MHC expression. #P < 0.05 versus control. Cont, control; Rap, rapamycin treatment.
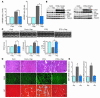
Figure 5
Coronary angiogenesis and angiogenic growth factor expression. (A) Left: Representative CD31 staining. Scale bar: 50 μm. Right: Capillary density. *P < 0.01. (B) Expressions of VEGF-A and Ang-2 in the heart. (C) Effects of rapamycin on capillary density and angiogenic growth factor expression in the acute phase. Left: Capillary density after 2 weeks of transgene induction. Right: Expression of VEGF-A and Ang-2 after 2 weeks of transgene induction. (D) Effects of rapamycin on capillary density and angiogenic growth factor expressions in the chronic phase. Left: Capillary density after 6 weeks of transgene induction. *P < 0.01. Right: Expression of VEGF-A and Ang-2 after 6 weeks of transgene induction.
Figure 6
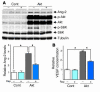
Induction of VEGF-A and Ang-2 by Akt in cultured adult cardiac myocytes. (A) Top: Representative Western blot analysis of Ang-2, Akt, and S6K. Bottom: Densitometric analysis of Ang-2 expression levels. *P < 0.01; #P < 0.05. (B) VEGF concentration in the culture media as measured by ELISA. *P < 0.01.
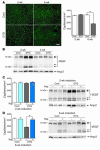
Figure 7
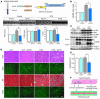
Inhibition of coronary angiogenesis results in impaired cardiac growth and contractile dysfunction. (A) Echocardiography. Top: Schematic illustrations of adenoviruses and experimental protocol. KDR, kinase domain insert–containing receptor. Middle: Representative M-mode recordings. Bottom: Echocardiographic parameters. *P < 0.01; #P < 0.05. (B) HW/BW ratio of control or DTG hearts treated with a control vector (Ad-cont) or adenoviral vector encoding Flk1-Fc (Ad-Flk). *P < 0.01. (C) Representative Western blot of Akt, S6K, VEGF-A, and Ang-2. (D) Histology of control or DTG hearts treated with Ad-cont or Ad-Flk. Scale bars: 50 μm. (E) Capillary density of control or DTG hearts treated with Ad-cont or Ad-Flk. *P < 0.01. (F) Cross-talk between cardiac myocytes and coronary vasculature during cardiac growth. Secretion of multiple angiogenic growth factors including VEGF and Ang-2 from cardiomyocytes is thought to be responsible for enhanced coronary angiogenesis during adaptive cardiac growth. Coronary vasculature, on the other hand, is thought to contribute to cardiac growth and the maintenance of contractile function.
Comment in
- Akt1 in the cardiovascular system: friend or foe?
O'Neill BT, Abel ED. O'Neill BT, et al. J Clin Invest. 2005 Aug;115(8):2059-64. doi: 10.1172/JCI25900. J Clin Invest. 2005. PMID: 16075047 Free PMC article.
Similar articles
- Adiponectin deficiency exacerbates cardiac dysfunction following pressure overload through disruption of an AMPK-dependent angiogenic response.
Shimano M, Ouchi N, Shibata R, Ohashi K, Pimentel DR, Murohara T, Walsh K. Shimano M, et al. J Mol Cell Cardiol. 2010 Aug;49(2):210-20. doi: 10.1016/j.yjmcc.2010.02.021. Epub 2010 Mar 4. J Mol Cell Cardiol. 2010. PMID: 20206634 Free PMC article. - Akt3 overexpression in the heart results in progression from adaptive to maladaptive hypertrophy.
Taniyama Y, Ito M, Sato K, Kuester C, Veit K, Tremp G, Liao R, Colucci WS, Ivashchenko Y, Walsh K, Shiojima I. Taniyama Y, et al. J Mol Cell Cardiol. 2005 Feb;38(2):375-85. doi: 10.1016/j.yjmcc.2004.12.002. Epub 2005 Jan 25. J Mol Cell Cardiol. 2005. PMID: 15698844 - Vascular endothelial growth factor blockade prevents the beneficial effects of β-blocker therapy on cardiac function, angiogenesis, and remodeling in heart failure.
Rengo G, Cannavo A, Liccardo D, Zincarelli C, de Lucia C, Pagano G, Komici K, Parisi V, Scala O, Agresta A, Rapacciuolo A, Perrone Filardi P, Ferrara N, Koch WJ, Trimarco B, Femminella GD, Leosco D. Rengo G, et al. Circ Heart Fail. 2013 Nov;6(6):1259-67. doi: 10.1161/CIRCHEARTFAILURE.113.000329. Epub 2013 Sep 12. Circ Heart Fail. 2013. PMID: 24029661 - Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure.
Oka T, Akazawa H, Naito AT, Komuro I. Oka T, et al. Circ Res. 2014 Jan 31;114(3):565-71. doi: 10.1161/CIRCRESAHA.114.300507. Circ Res. 2014. PMID: 24481846 Review. - Growth hormone, acromegaly, and heart failure: an intricate triangulation.
Saccà L, Napoli R, Cittadini A. Saccà L, et al. Clin Endocrinol (Oxf). 2003 Dec;59(6):660-71. doi: 10.1046/j.1365-2265.2003.01780.x. Clin Endocrinol (Oxf). 2003. PMID: 14974906 Review.
Cited by
- The programming of cardiac hypertrophy in the offspring by maternal obesity is associated with hyperinsulinemia, AKT, ERK, and mTOR activation.
Fernandez-Twinn DS, Blackmore HL, Siggens L, Giussani DA, Cross CM, Foo R, Ozanne SE. Fernandez-Twinn DS, et al. Endocrinology. 2012 Dec;153(12):5961-71. doi: 10.1210/en.2012-1508. Epub 2012 Oct 15. Endocrinology. 2012. PMID: 23070543 Free PMC article. - Angiogenesis in the infarcted myocardium.
Cochain C, Channon KM, Silvestre JS. Cochain C, et al. Antioxid Redox Signal. 2013 Mar 20;18(9):1100-13. doi: 10.1089/ars.2012.4849. Epub 2012 Sep 25. Antioxid Redox Signal. 2013. PMID: 22870932 Free PMC article. Review. - Ongoing controversies surrounding cardiac remodeling: is it black and white-or rather fifty shades of gray?
Spaich S, Katus HA, Backs J. Spaich S, et al. Front Physiol. 2015 Jul 22;6:202. doi: 10.3389/fphys.2015.00202. eCollection 2015. Front Physiol. 2015. PMID: 26257654 Free PMC article. Review. - Regulation of cardiac hypertrophic signaling by prolyl isomerase Pin1.
Toko H, Konstandin MH, Doroudgar S, Ormachea L, Joyo E, Joyo AY, Din S, Gude NA, Collins B, Völkers M, Thuerauf DJ, Glembotski CC, Chen CH, Lu KP, Müller OJ, Uchida T, Sussman MA. Toko H, et al. Circ Res. 2013 Apr 26;112(9):1244-52. doi: 10.1161/CIRCRESAHA.113.301084. Epub 2013 Mar 13. Circ Res. 2013. PMID: 23487407 Free PMC article. - Evaluation of Tyrosine Kinase Inhibitors Loaded Injectable Hydrogels for Improving Connexin43 Gap Junction Intercellular Communication.
Zheng L, Shi W, Liu B, Duan B, Sorgen PL. Zheng L, et al. ACS Appl Mater Interfaces. 2024 Jan 17;16(2):1985-1998. doi: 10.1021/acsami.3c10923. Epub 2024 Jan 4. ACS Appl Mater Interfaces. 2024. PMID: 38175743 Free PMC article.
References
- Braunwald, E., Colucci, W.S., and Grossman, W. 1997. Clinical aspects of heart failure: high-output heart failure; pulmonary edema. In Heart disease: a textbook of cardiovascular medicine. E. Braunwald, editor. W. B. Sanders. Philadelphia, Pennsylvania, USA. 445–470.
- American Heart Association. 2004. Heart diseases and stroke statistics: 2005 update. American Heart Association. Dallas, Texas, USA. http://www.americanheart.org/presenter.jhtml?identifier=1928.
- Olivetti G, Capasso JM, Meggs LG, Sonnenblick EH, Anversa P. Cellular basis of chronic ventricular remodeling after myocardial infarction in rats. Circ. Res. 1991;68:856–869. - PubMed
- Gerdes AM, et al. Structural remodeling of cardiac myocytes in patients with ischemic cardiomyopathy. Circulation. 1992;86:426–430. - PubMed
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N. Engl. J. Med. 1990;322:1561–1566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL77774/HL/NHLBI NIH HHS/United States
- HL66957/HL/NHLBI NIH HHS/United States
- P01 HL066957/HL/NHLBI NIH HHS/United States
- R01 AG017241/AG/NIA NIH HHS/United States
- AR40197/AR/NIAMS NIH HHS/United States
- R01 AG015052/AG/NIA NIH HHS/United States
- AG15052/AG/NIA NIH HHS/United States
- R37 AG015052/AG/NIA NIH HHS/United States
- R01 AR040197/AR/NIAMS NIH HHS/United States
- R01 HL077774/HL/NHLBI NIH HHS/United States
- AG17241/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous