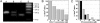Interferon-alpha and -beta differentially regulate osteoclastogenesis: role of differential induction of chemokine CXCL11 expression - PubMed (original) (raw)
Interferon-alpha and -beta differentially regulate osteoclastogenesis: role of differential induction of chemokine CXCL11 expression
Luiz F Leomil Coelho et al. Proc Natl Acad Sci U S A. 2005.
Abstract
In humans, type I interferon (IFN) is a family of 17 cytokines, among which the alpha subtypes and the beta subtype are differentially expressed. It has been suggested that IFN-beta activates a specific signaling cascade in addition to those activated by all type I IFNs. Nevertheless, no true biological relevance for a differential activity of alpha and beta IFN subtypes has been identified so far. Because type I IFNs are critical for the regulation of osteoclastogenesis in mice, we have compared the effect of IFN-alpha2 and IFN-beta on the differentiation of human monocytes into osteoclasts. Primary monocytes undergoing osteoclastic differentiation are highly and equally sensitive to both alpha2 and beta IFNs as determined by measuring the induction levels of several IFN-stimulated genes. However, IFN-beta was 100-fold more potent than the alpha2 subtype at inhibiting osteoclastogenesis. Expression profiling of the genes differentially regulated by IFN-alpha2 and IFN-beta in this cellular system revealed the chemokine CXCL11 as the only IFN-induced gene differentially up-regulated by IFN-beta. We show that recombinant CXCL11 by itself inhibits osteoclastic differentiation. These results indicate that autocrine-acting CXCL11 mediates, at least in part, the regulations of osteoclastogenesis by type I IFNs.
Figures
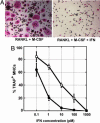
Fig. 1.
IFN-α2 and IFN-β exhibit a 100-fold difference in their specific activities toward the inhibition of the differentiation of monocytes in osteoclasts. Freshly purified monocytes from human blood donors were cultured in the presence of sRANKL and M-CSF for 2 days, and with different concentrations of IFN-α2 or IFN-β for an additional 4–6 days. Cells were then fixed and stained for TRAP and nuclei. (A) Photomicrographs of IFN-free culture (Left) or culture with 1 nM IFN (Right). (B) Quantification of the number of TRAP+ MNCs in IFN-α2-treated culture (○) or IFN-β treated culture (•). Each point represents the mean osteoclast number relative to IFN-free cultures ± SEM from 5 to 29 blood donors. The osteoclastic differentiation is inhibited by 30% with 1 pM IFN-α2, 80% with 1 pM IFN-β, 60% with 10 pM IFN-α2, and 95% with 10 pM IFN-β.
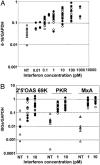
Fig. 2.
IFN-α2 and IFN-β exhibit the same specific activities for the induction of the expression of IFN-stimulated genes. Freshly purified monocytes from human blood donors were cultured in the presence of sRANKL and M-CSF for 2 days, and then left untreated (▵) or treated for 4 h with IFN-α2 (○) or IFN β (•). RNAs were then extracted and the levels of 6-16 (A) or 2′–5′ oligoadenylate synthetase, PKR, and MxA (B) transcripts were measured by quantitative RT-PCR. Each point in the vertical represents a different blood donor.
Fig. 3.
CXCL11 is the only ISG differentially up-regulated by IFN-β compared with IFN-α2 in monocytes purified from several blood donors. Candidate genes identified as preferentially up-regulated by 1 pM IFN-β in the gene array study (Table 1) were analyzed by quantitative RT-PCR. Freshly purified monocytes from human blood donors were cultured in the presence of sRANKL and M-CSF for 2 days, and then left untreated (▵) or treated for 4 h with IFN-α2 (○) or IFN-β (•). RNAs were then extracted and the levels of ISG20 (A), GBP5 (B), and CXCL11 transcripts (C) were quantified by quantitative RT-PCR. Each point in the vertical represents a different blood donor. Samples used for the gene array study are indicated by arrows. Statistical significance of the difference of the expression levels induced by IFN-α2 or IFN-β have been analyzed by the Mann–Whitney test. NS, nonsignificant. The median of the ratio of CXCL11 induced by IFN-β/CXCL11 induced by IFN-α2 is 4.2 for IFNs at 1 pM and 1.5 for IFNs at 10 pM.
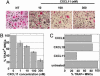
Fig. 4.
CXCL11 but not CXCL9 or CXCL10 inhibits osteoclastic differentiation. Freshly purified monocytes from human blood donors were cultured in the presence of sRANKL and M-CSF for 2 days, and with different concentrations of the chemokine for an additional 4–6 days. Cells were then fixed and stained for TRAP and nuclei. (A) Photomicrographs of CXCL11-treated cultures. (B) Quantification of the number of TRAP+ MNCs in CXCL11-treated cultures; mean osteoclast number relative to untreated cultures ± SEM from eight blood donors. (C) Comparison of the effect of 100 nM CXCL11, CXCL9, and CXCL10 on the inhibition of osteoclastic differentiation.
Fig. 5.
The inhibitory effect of CXCL11 on osteoclastogenesis is independent of CCR5. (A) Identification of monocytes heterozygous (lane B) or homozygous (lane C) for the CCR5 Δ32 mutation. Lanes: A, wild type; D, molecular weight marker in base pairs. (B and C) Inhibitory activity of CXCL11 (B), IFN-α2(C, gray bars) and IFN-β (C, black bars) on osteoclastic differentiation of monocytes homozygous for the CCR5 Δ32 mutation. Cellular differentiation assay and analysis were performed as for Figs. 1 and 4.
Similar articles
- Interferon-α, -β and -γ induce CXCL11 secretion in human thyrocytes: modulation by peroxisome proliferator-activated receptor γ agonists.
Antonelli A, Ferrari SM, Mancusi C, Mazzi V, Pupilli C, Centanni M, Ferri C, Ferrannini E, Fallahi P. Antonelli A, et al. Immunobiology. 2013 May;218(5):690-5. doi: 10.1016/j.imbio.2012.08.267. Epub 2012 Aug 9. Immunobiology. 2013. PMID: 22944249 - Up-regulation of the interferon gamma (IFN-gamma)-inducible chemokines IFN-inducible T-cell alpha chemoattractant and monokine induced by IFN-gamma and of their receptor CXC receptor 3 in human renal cell carcinoma.
Suyama T, Furuya M, Nishiyama M, Kasuya Y, Kimura S, Ichikawa T, Ueda T, Nikaido T, Ito H, Ishikura H. Suyama T, et al. Cancer. 2005 Jan 15;103(2):258-67. doi: 10.1002/cncr.20747. Cancer. 2005. PMID: 15578685 - Inquiring into the differential action of interferons (IFNs): an IFN-alpha2 mutant with enhanced affinity to IFNAR1 is functionally similar to IFN-beta.
Jaitin DA, Roisman LC, Jaks E, Gavutis M, Piehler J, Van der Heyden J, Uze G, Schreiber G. Jaitin DA, et al. Mol Cell Biol. 2006 Mar;26(5):1888-97. doi: 10.1128/MCB.26.5.1888-1897.2006. Mol Cell Biol. 2006. PMID: 16479007 Free PMC article. - The Role of CXCL11 and its Receptors in Cancer: Prospective but Challenging Clinical Targets.
Wang J, Ouyang X, Zhu W, Yi Q, Zhong J. Wang J, et al. Cancer Control. 2024 Jan-Dec;31:10732748241241162. doi: 10.1177/10732748241241162. Cancer Control. 2024. PMID: 38533911 Free PMC article. Review. - [Interferon and bone].
Miyamoto T. Miyamoto T. Clin Calcium. 2015 Nov;25(11):1653-8. Clin Calcium. 2015. PMID: 26503870 Review. Japanese.
Cited by
- The causal role of circulating inflammatory markers in osteoporosis: a bidirectional Mendelian randomized study.
Dong Q, Wu J, Zhang H, Luo L, Wu W. Dong Q, et al. Front Immunol. 2024 Jul 18;15:1412298. doi: 10.3389/fimmu.2024.1412298. eCollection 2024. Front Immunol. 2024. PMID: 39091505 Free PMC article. - Interferon beta induces mature dendritic cell apoptosis through caspase-11/caspase-3 activation.
Yen JH, Ganea D. Yen JH, et al. Blood. 2009 Aug 13;114(7):1344-54. doi: 10.1182/blood-2008-12-196592. Epub 2009 Jun 16. Blood. 2009. PMID: 19531658 Free PMC article. - Assessment of bone remodelling in childhood-onset systemic lupus erythematosus.
Baker-LePain JC, Nakamura MC, Shepherd J, von Scheven E. Baker-LePain JC, et al. Rheumatology (Oxford). 2011 Mar;50(3):611-9. doi: 10.1093/rheumatology/keq307. Epub 2010 Nov 23. Rheumatology (Oxford). 2011. PMID: 21098573 Free PMC article. - Osteoimmunology and the influence of pro-inflammatory cytokines on osteoclasts.
Zupan J, Jeras M, Marc J. Zupan J, et al. Biochem Med (Zagreb). 2013;23(1):43-63. doi: 10.11613/bm.2013.007. Biochem Med (Zagreb). 2013. PMID: 23457765 Free PMC article. Review. - Differential activity of type I interferon subtypes for dendritic cell differentiation.
Garcin G, Bordat Y, Chuchana P, Monneron D, Law HK, Piehler J, Uzé G. Garcin G, et al. PLoS One. 2013;8(3):e58465. doi: 10.1371/journal.pone.0058465. Epub 2013 Mar 5. PLoS One. 2013. PMID: 23472200 Free PMC article.
References
- Gresser, I. & Belardelli, F. (2002) Cytokine Growth Factor Rev. 13, 111-118. - PubMed
- Santini, S. M., Di Pucchio, T., Lapenta, C., Parlato, S., Logozzi, M. & Belardelli, F. (2002) J. Interferon Cytokine Res. 22, 1071-1080. - PubMed
- Chen, J., Baig, E. & Fish, E. N. (2004) J. Interferon Cytokine Res. 24, 687-698. - PubMed
- Langer, J. A., Cutrone, E. C. & Kotenko, S. (2004) Cytokine Growth Factor Rev. 15, 33-48. - PubMed
- Platanias, L. C. & Fish, E. N. (1999) Exp. Hematol. (Charlottesville, Va) 27, 1583-1592. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous