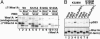Cyclin-dependent kinase (CDK) phosphorylation destabilizes somatic Wee1 via multiple pathways - PubMed (original) (raw)
Cyclin-dependent kinase (CDK) phosphorylation destabilizes somatic Wee1 via multiple pathways
Nobumoto Watanabe et al. Proc Natl Acad Sci U S A. 2005.
Abstract
At the onset of M phase, the activity of somatic Wee1 (Wee1A), the inhibitory kinase for cyclin-dependent kinase (CDK), is down-regulated primarily through proteasome-dependent degradation after ubiquitination by the E3 ubiquitin ligase SCF(beta-TrCP). The F-box protein beta-TrCP (beta-transducin repeat-containing protein), the substrate recognition component of the ubiquitin ligase, binds to its substrates through a conserved binding motif (phosphodegron) containing two phosphoserines, DpSGXXpS. Although Wee1A lacks this motif, phosphorylation of serines 53 and 123 (S53 and S123) of Wee1A by polo-like kinase 1 (Plk1) and CDK, respectively, are required for binding to beta-TrCP. The sequence surrounding phosphorylated S53 (DpSAFQE) is similar to the conserved beta-TrCP-binding motif; however, the role of S123 phosphorylation (EEGFGSSpSPVK) in beta-TrCP binding was not elucidated. In the present study, we show that phosphorylation of S123 (pS123) by CDK promoted the binding of Wee1A to beta-TrCP through three independent mechanisms. The pS123 not only directly interacted with basic residues in the WD40 repeat domain of beta-TrCP but also primed phosphorylation by two independent protein kinases, Plk1 and CK2 (formerly casein kinase 2), to create two phosphodegrons on Wee1A. In the case of Plk1, S123 phosphorylation created a polo box domain-binding motif (SpSP) on Wee1A to accelerate phosphorylation of S53 by Plk1. CK2 could phosphorylate S121, but only if S123 was phosphorylated first, thereby generating the second beta-TrCP-binding site (EEGFGpS121). Using a specific inhibitor of CK2, we showed that the phosphorylation-dependent degradation of Wee1A is important for the proper onset of mitosis.
Figures
Fig. 1.
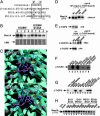
Phosphorylated S123 in Wee1A interacts with the WD40 domain of β-TrCP. (A) Two phosphodegrons (PD53 and PD123) in the Wee1A sequence and a phosphodegron in β-catenin are aligned with the consensus binding sequence (boxed) for β-TrCP. The important amino acids in Wee1A for binding to β-TrCP are underlined (1). (B) HeLa cells were transfected with kinase-negative Wee1A (K328M) or its S123A mutant. Two days after transfection, cells were treated with nocodazole (0.1 μg/ml) for 21 h. Cycloheximide (10 μg/ml) was then added, and the stability of Wee1A was examined by immunoblotting at the times indicated. (C) Structures of the phosphodegron peptide of β-catenin (Upper) and PD123 phosphopeptide of Wee1A (Lower) bound to the β-TrCP WD40 domain. The β-TrCP WD40 domain is shown in light blue, with its side chains in dark blue, and the phosphodegron peptides of β-catenin or Wee1A are in purple [phosphate groups on serines are shown in green (P) and red (O)]. Hydrogen bonds are represented by yellow dotted lines. The crystal structure of the interface between the WD40 domain and phosphodegron peptide of β-catenin is as published in ref. , although the numberings of the WD40 domain are for β-TrCP2, whereas the interface between the WD40 domain and PD123 is a representative model structure calculated by an energy minimization using
sybyl
6.91 with the Kollman
amber
force field (30). The calculated intermolecular interaction energy values between β-TrCP and each of the Wee1A peptides PS121PS123, PS121S123, S121PS123, and S121S123 were –2.33 ± 0.33, –1.96 ± 0.13, –1.79 ± 0.37, and –1.38 ± 0.26 × 103 kcal/mol, respectively; those between R383M and R404M mutated β-TrCP and PS121PS123 peptides were –1.98 ± 0.21 and –2.09 ± 0.29 × 103 kcal/mol, respectively. (D) Wee1A lacking 71 amino acids from the N terminus (Δ71Wee1A) was coexpressed with HA-tagged β-TrCP2 or its R383M mutant in HtTA-1 cells. Cell lysates (10% of total input) and αHA immunoprecipitates of the lysates were analyzed by immunoblotting. HA-β-TrCP2 is hard to detect in αHA immunoprecipitates, because it migrates at a similar position to that of Ig heavy chain. (E) HA-taggedβ-TrCP2 or its R383M mutant was coexpressed with Myc-tagged β-catenin and axin in HtTA-1 cells and immunoprecipitated as in D.β-Catenin bound to HA-β-TrCP2 was detected by using αMyc antibody. (F and G) Peptides as indicated were coupled to Sepharose beads. The beads were mixed with HtTA-1 cell lysates expressing HA-tagged β-TrCP2 and washed. Cell lysates (10% of total input) and bound proteins were examined by immunoblotting with αHA antibody. (H) Δ71Wee1A was coexpressed with HA-tagged β-TrCP2 in HtTA-1 cells. The ability of peptides to abolish the association of Wee1A and β-TrCP2 was examined by the addition of peptides at the indicated concentration to the cell lysates before the addition of αHA antibody in the coimmunoprecipitation assay as in D.
Fig. 2.
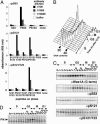
Phosphorylation of S53, S121, and S123 in synchronized HeLa cells. (A) Serially diluted affinity-purified antibodies as indicated were added to wells of ELISA plates, which are coated with each phosphopeptide as shown in the horizontal axis. Bound antibodies were quantified by the peroxidase activity of horseradish peroxidase-conjugated secondary antibody. (B) HeLa cells were synchronized at the G1/S boundary and released. Cell cycle progression was monitored by DNA content determined by FACS analysis. (C) Levels of Wee1A or phosphorylation at S53, S121, and S121 and S123 was examined by immunoblotting of total cell lysates [equivalent to the same number of cells at the G1/S border (time 0); the levels of total proteins were confirmed by Coomassie blue staining of the filter (not shown)] from each time point. (D) Levels of Plk1 were examined by immunoblotting of total cell lysates from each time point as in C.
Fig. 3.
S123 phosphorylation creates a PBD-binding motif and accelerates S53 phosphorylation by Plk1. (A) WT GST-PBD (WT) and its pincer sequence mutant form (Mt, H538A/K540M) were expressed in bacteria. The bacterial lysates were mixed with HeLa cell lysates expressing WT or mutant forms of Δ71Wee1A. Cell lysates (In, 50% of total input is shown) and GST-pulled-down proteins (Bind) were analyzed by immunoblotting with αWee1A antiserum. Endogenous HeLa cell Wee1A also bound detectably to WT PBD. Asterisks indicate nonspecific signals derived from bacterial lysates. (B) Kinase-negative mutant Wee1A (K328M) or its S123A derivative was expressed in HeLa cells, immunopurified, and phosphorylated by purified cyclin B1/Cdc2 and/or Plk1 as indicated. Phosphorylation of S53 and the level of Wee1A were analyzed on the same filter by sequentially immunoprobing with αpS53 antibody and α-C-terminal antiserum, respectively.
Fig. 4.
Phosphorylation at S123 promotes S121 phosphorylation by CK2, which is essential for the proper onset of Wee1A degradation and M phase. (A) Synthetic PD123 peptide [(C)EEEGFG_SSS_PVKSPAAP] and its phosphorylated versions (status of the three italicized serines is indicated) were phosphorylated by CK2 and cyclin B1/Cdc2 and analyzed by electrophoresis on a thin-layer cellulose plate at pH 3.5 toward the anode (bottom). The positions of singly (1xP) and doubly (2xP) phosphorylated peptides are shown. Doubly phosphorylated peptides migrated as doublets for unknown reasons. (B) PD123 peptide or its derivatives (phosphoserine or alanine substitution of three serines italicized in A are indicated) were phosphorylated by CK2 and analyzed as in A. (C) Kinase-negative mutant Wee1A (K328M) was expressed in HeLa cells, immunopurified, and phosphorylated by cyclin B1/Cdc2 and/or CK2α in the presence of 100 μM ATP. In some reactions, TBB was added at a final concentration as indicated. After the reaction and Western transfer, phosphorylation of S121 and level of Wee1A were analyzed on the same filter by sequentially immunoprobing with αpS121 antibody and α-C-terminal antiserum, respectively. (D) HeLa cells were synchronized as in Fig. 2 and cultured in the absence or presence of TBB (75 μM, added at 3 h after the release). The level of Wee1A and phosphorylation of S121 were analyzed by immunoblotting as in Fig. 2_C_. (E) HeLa cells were transfected with siRNA for CK2α and/or α′. Two days after transfection, the levels of Wee1A, CK2α, and CK2α′ were analyzed by immunoblotting. Coomassie blue (CBB) staining of the filter is also shown (arrowhead indicates the position of Wee1A). (F) Effect of TBB (75μM, added at 3 h after the release) on the cell cycle progression was monitored by DNA content determined by FACS analysis. DNA contents of G1 (2C) and G2/M (4C) cells are indicated by triangles. (G) Effect of TBB (75 μM, added at 3 h after the release) on the onset of M phase was monitored by H1 histone kinase activity of cyclin B1 immunoprecipitates. (H) TBB (75 μM, added at 3 h after the release)-treated cells (Center) appear “angular” at 10 h after the release, when most of the control cells (Left) were rounded up. TBB-treated cells started to round up and divide at ≈12 h (not shown). Nonsynchronized control cells are also shown (Right). (Scale bars, 100 μm.)
Fig. 5.
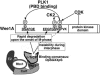
Phosphorylation at S123 by CDK promotes the binding of β-TrCP to Wee1A via three independent pathways. During the S and G2 phases, S123 is phosphorylated by a CDK (possibly CDK2). pS123 primes CK2 to phosphorylate S121, resulting in creation of a β-TrCP phosphodegron (EEGFGpS121) that is responsible for the instability of Wee1A during interphase. PD123 binding involves interaction of pS123 with basic residues in the WD40 repeat domain of β-TrCP in a manner unique to Wee1A (dotted line). At the onset of M phase, when activated Plk1 accumulates, Plk1 binds to Wee1A at the PBD-binding motif surrounding pS123 (SpSP) by means of its PBD and phosphorylates S53, resulting in generation of the second phosphodegron (DpSAFQE). These two phosphodegrons act cooperatively to promote β-TrCP binding and the turnover of Wee1A, which is important for the proper onset of M phase.
Similar articles
- M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta-TrCP.
Watanabe N, Arai H, Nishihara Y, Taniguchi M, Watanabe N, Hunter T, Osada H. Watanabe N, et al. Proc Natl Acad Sci U S A. 2004 Mar 30;101(13):4419-24. doi: 10.1073/pnas.0307700101. Epub 2004 Mar 22. Proc Natl Acad Sci U S A. 2004. PMID: 15070733 Free PMC article. - Ability of CK2beta to selectively regulate cellular protein kinases.
Olsen BB, Guerra B. Olsen BB, et al. Mol Cell Biochem. 2008 Sep;316(1-2):115-26. doi: 10.1007/s11010-008-9817-2. Epub 2008 Jun 17. Mol Cell Biochem. 2008. PMID: 18560763 - The regulatory beta-subunit of protein kinase CK2 regulates cell-cycle progression at the onset of mitosis.
Yde CW, Olsen BB, Meek D, Watanabe N, Guerra B. Yde CW, et al. Oncogene. 2008 Aug 28;27(37):4986-97. doi: 10.1038/onc.2008.146. Epub 2008 May 12. Oncogene. 2008. PMID: 18469858 - Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/β-TrCP.
Cuadrado A. Cuadrado A. Free Radic Biol Med. 2015 Nov;88(Pt B):147-157. doi: 10.1016/j.freeradbiomed.2015.04.029. Epub 2015 Apr 30. Free Radic Biol Med. 2015. PMID: 25937177 Review. - Guiding Mitotic Progression by Crosstalk between Post-translational Modifications.
Cuijpers SAG, Vertegaal ACO. Cuijpers SAG, et al. Trends Biochem Sci. 2018 Apr;43(4):251-268. doi: 10.1016/j.tibs.2018.02.004. Epub 2018 Feb 24. Trends Biochem Sci. 2018. PMID: 29486978 Review.
Cited by
- Expression of constitutively active CDK1 stabilizes APC-Cdh1 substrates and potentiates premature spindle assembly and checkpoint function in G1 cells.
Ma Y, Yuan X, Wyatt WR, Pomerening JR. Ma Y, et al. PLoS One. 2012;7(3):e33835. doi: 10.1371/journal.pone.0033835. Epub 2012 Mar 29. PLoS One. 2012. PMID: 22479455 Free PMC article. - Human prostate epithelium lacks Wee1A-mediated DNA damage-induced checkpoint enforcement.
Kiviharju-af Hällström TM, Jäämaa S, Mönkkönen M, Peltonen K, Andersson LC, Medema RH, Peehl DM, Laiho M. Kiviharju-af Hällström TM, et al. Proc Natl Acad Sci U S A. 2007 Apr 24;104(17):7211-6. doi: 10.1073/pnas.0609299104. Epub 2007 Apr 12. Proc Natl Acad Sci U S A. 2007. PMID: 17431037 Free PMC article. - A new wave of innovations within the DNA damage response.
Li Q, Qian W, Zhang Y, Hu L, Chen S, Xia Y. Li Q, et al. Signal Transduct Target Ther. 2023 Sep 8;8(1):338. doi: 10.1038/s41392-023-01548-8. Signal Transduct Target Ther. 2023. PMID: 37679326 Free PMC article. Review. - CHK1 Inhibitor Blocks Phosphorylation of FAM122A and Promotes Replication Stress.
Li F, Kozono D, Deraska P, Branigan T, Dunn C, Zheng XF, Parmar K, Nguyen H, DeCaprio J, Shapiro GI, Chowdhury D, D'Andrea AD. Li F, et al. Mol Cell. 2020 Nov 5;80(3):410-422.e6. doi: 10.1016/j.molcel.2020.10.008. Epub 2020 Oct 26. Mol Cell. 2020. PMID: 33108758 Free PMC article. - Integrated functional, gene expression and genomic analysis for the identification of cancer targets.
Iorns E, Lord CJ, Grigoriadis A, McDonald S, Fenwick K, Mackay A, Mein CA, Natrajan R, Savage K, Tamber N, Reis-Filho JS, Turner NC, Ashworth A. Iorns E, et al. PLoS One. 2009;4(4):e5120. doi: 10.1371/journal.pone.0005120. Epub 2009 Apr 9. PLoS One. 2009. PMID: 19357772 Free PMC article.
References
- Van Vugt, M. A., Bras, A. & Medema, R. H. (2004) Mol. Cell 15, 799–811. - PubMed
- Barr, F. A., Sillje, H. H. & Nigg, E. A. (2004) Nat. Rev. Mol. Cell. Biol. 5, 429–440. - PubMed
- Lane, H. A. & Nigg, E. A. (1997) Trends Cell Biol. 7, 63–68. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous