Anti-Fas mAb-induced apoptosis and cytolysis of airway tissue eosinophils aggravates rather than resolves established inflammation - PubMed (original) (raw)
Anti-Fas mAb-induced apoptosis and cytolysis of airway tissue eosinophils aggravates rather than resolves established inflammation
Lena Uller et al. Respir Res. 2005.
Abstract
Background: Fas receptor-mediated eosinophil apoptosis is currently forwarded as a mechanism resolving asthma-like inflammation. This view is based on observations in vitro and in airway lumen with unknown translatability to airway tissues in vivo. In fact, apoptotic eosinophils have not been detected in human diseased airway tissues whereas cytolytic eosinophils abound and constitute a major mode of degranulation of these cells. Also, Fas receptor stimulation may bypass the apoptotic pathway and directly evoke cytolysis of non-apoptotic cells. We thus hypothesized that effects of anti-Fas mAb in vivo may include both apoptosis and cytolysis of eosinophils and, hence, that established eosinophilic inflammation may not resolve by this treatment.
Methods: Weeklong daily allergen challenges of sensitized mice were followed by airway administration of anti-Fas mAb. BAL was performed and airway-pulmonary tissues were examined using light and electron microscopy. Lung tissue analysis for CC-chemokines, apoptosis, mucus production and plasma exudation (fibrinogen) were performed.
Results: Anti-Fas mAb evoked apoptosis of 28% and cytolysis of 4% of eosinophils present in allergen-challenged airway tissues. Furthermore, a majority of the apoptotic eosinophils remained unengulfed and eventually exhibited secondary necrosis. A striking histopathology far beyond the allergic inflammation developed and included degranulated eosinophils, neutrophilia, epithelial derangement, plasma exudation, mucus-plasma plugs, and inducement of 6 CC-chemokines. In animals without eosinophilia anti-Fas evoked no inflammatory response.
Conclusion: An efficient inducer of eosinophil apoptosis in airway tissues in vivo, anti-Fas mAb evoked unprecedented asthma-like inflammation in mouse allergic airways. This outcome may partly reflect the ability of anti-Fas to evoke direct cytolysis of non-apoptotic eosinophils in airway tissues. Additionally, since most apoptotic tissue eosinophils progressed into the pro-inflammatory cellular fate of secondary necrosis this may also explain the aggravated inflammation. Our data indicate that Fas receptor mediated eosinophil apoptosis in airway tissues in vivo may cause severe disease exacerbation due to direct cytolysis and secondary necrosis of eosinophils.
Figures
Figure 1
Study design. All animals were immunized with OVA and 14 days later exposed to aerosol challenge with OVA for 7 days to establish tissue and lumen eosinophilia. Treatment with anti-Fas mAb or isotype control IgG was administered intra-nasally at day 22 and outcome measurements including BAL and tissue sampling were made at 8 and 24 h after treatment.
Figure 2
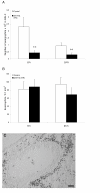
After eosinophilia had been established in the immunized mice anti-Fas mAb or isotype control Ab was administered locally to the lungs followed by BAL and tissue sampling at 8 and 24 hours. The number of eosinophils in airway lumen (A) and airway tissue (B) was quantified as described in detail in the methods section. White bars represent mice given control Ab and black bars mice given anti-Fas mAb. Error bars indicate the standard error of the mean for each group of mice (n = 8, ** = p < 0.01). Peribronchial eosinophilia induced by the OVA challenges is shown in (C).
Figure 3
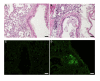
Representative light micrographs of mouse lung tissue using Htx-staining in control (A) and anti-Fas mAb treated animals (B) at 24 h. Htx-staining shows dark condensed (pycnotic) nuclei of eosinophils and disturbed epithelial lining. Very few TUNEL-positive apoptotic cells were present in control treated animals (C) whereas a large number of TUNEL-stained cells was detected in anti-Fas mAb treated animals (D), almost all of which were shown to be apoptotic eosinophils by double chromotrope 2R and TUNEL staining (see also Figure 4).
Figure 4
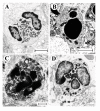
Transmission electron micrographs of lung tissue from mice with OVA-induced eosinophilia. Control mice treated with the isotype control Ab showed no sign of eosinophil apoptosis at 8 h (A) and 24 h (not shown). In mice treated with anti-Fas mAb there were numerous apoptotic eosinophils in the lung tissues at both 8 and 24 hours after treatment (B and C, respectively). The apoptotic eosinophils were rarely engulfed although macrophages (labeled M) commonly occurred in the tissue (B). By 24 h a majority of the apoptotic eosinophils exhibited signs of secondary necrosis and severe inflammation was recorded including neutrophil infiltration (arrow) and derangement of the epithelial lining (labeled E).
Figure 5
These micrographs illustrate characteristic eosinophil phenotypes present in mouse airways in this study: (A) viable non-degranulating eosinophil, the only phenotype found in lung tissues of allergen challenged animals; (B) apoptotic eosinophil exhibiting nuclear condensation, cell shrinkage, and an intact cell membrane; (C) an apoptotic eosinophil exhibiting secondary necrosis involving cell membrane rupture and piecemeal degranulation; (D) a cytolytic eosinophil exhibiting chromatolysis, cell membrane rupture, and spilling of electron-dense specific granules into the tissue (arrow).
Figure 6
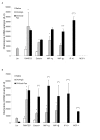
CC-chemokine mRNA expression 8 h (A) and 24 h (B) after treatment with anti-Fas mAb or isotype control (IgG). Two days post allergen challenge expression of 5 different CC-chemokines in the lung was up-regulated compared to immunized control animals receiving saline challenges. Treatment with anti-Fas mAb post allergen challenge further increased the expression of eotaxin MIP-1α, and MIP -1β, and additionally induced the expression of IP-10 and MCP-1. Data are mean ± SEM. **p < 0.01 indicates differences between OVA and saline treatments. §§ p < 0.01 indicates difference between anti-Fas mAb treated and control-treated OVA-challenged animals.
Figure 7
Observations in allergen challenged animals demonstrating anti-Fas-induced mucus-exudate plugs (A,C,D,E) compared to control isotype antibody (IgG) treatment (A,B). The occurrence of mucus-exudate plugs is expressed as percentage affected airways in each tissue section in control mice (white bars) and in mice treated with anti-Fas mAb (black bars) at 8 and 24 hours (A). Bars indicate the standard error of the mean for each group of animals (n = 8, ** = p < 0.01). Histochemical staining with periodic acid-Schiff reagent (PAS) illustrated mucus-containing cells (B,C) and so did the transmission electron micrograph (D). Tethered secretions and lumen plugs occurred foremost in anti-Fas treated airways (C,D;E). Fibrinogen immuno-reactivity was distributed in the mucus plugs exclusively in anti-Fas treated airways (E).
Similar articles
- Activation of the Fas receptor on lung eosinophils leads to apoptosis and the resolution of eosinophilic inflammation of the airways.
Tsuyuki S, Bertrand C, Erard F, Trifilieff A, Tsuyuki J, Wesp M, Anderson GP, Coyle AJ. Tsuyuki S, et al. J Clin Invest. 1995 Dec;96(6):2924-31. doi: 10.1172/JCI118364. J Clin Invest. 1995. PMID: 8675664 Free PMC article. - Degranulation status of airway tissue eosinophils in mouse models of allergic airway inflammation.
Malm-Erjefält M, Persson CG, Erjefält JS. Malm-Erjefält M, et al. Am J Respir Cell Mol Biol. 2001 Mar;24(3):352-9. doi: 10.1165/ajrcmb.24.3.4357. Am J Respir Cell Mol Biol. 2001. PMID: 11245636 - Effects of steroid treatment on lung CC chemokines, apoptosis and transepithelial cell clearance during development and resolution of allergic airway inflammation.
Uller L, Lloyd CM, Rydell-Törmänen K, Persson CG, Erjefält JS. Uller L, et al. Clin Exp Allergy. 2006 Jan;36(1):111-21. doi: 10.1111/j.1365-2222.2006.02396.x. Clin Exp Allergy. 2006. PMID: 16393273 Free PMC article. - Granulocyte apoptosis and its role in the resolution and control of lung inflammation.
Haslett C. Haslett C. Am J Respir Crit Care Med. 1999 Nov;160(5 Pt 2):S5-11. doi: 10.1164/ajrccm.160.supplement_1.4. Am J Respir Crit Care Med. 1999. PMID: 10556161 Review. - Role of eosinophils in airway inflammation of chronic obstructive pulmonary disease.
Tashkin DP, Wechsler ME. Tashkin DP, et al. Int J Chron Obstruct Pulmon Dis. 2018 Jan 17;13:335-349. doi: 10.2147/COPD.S152291. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 29403271 Free PMC article. Review.
Cited by
- Apoptosis of Eosinophil Granulocytes.
Zustakova M, Kratochvilova L, Slama P. Zustakova M, et al. Biology (Basel). 2020 Dec 10;9(12):457. doi: 10.3390/biology9120457. Biology (Basel). 2020. PMID: 33321726 Free PMC article. Review. - Interferon-β deficiency at asthma exacerbation promotes MLKL mediated necroptosis.
Cerps SC, Menzel M, Mahmutovic Persson I, Bjermer L, Akbarshahi H, Uller L. Cerps SC, et al. Sci Rep. 2018 Mar 9;8(1):4248. doi: 10.1038/s41598-018-22557-6. Sci Rep. 2018. PMID: 29523863 Free PMC article. - Regulation of spontaneous eosinophil apoptosis-a neglected area of importance.
Ilmarinen P, Moilanen E, Kankaanranta H. Ilmarinen P, et al. J Cell Death. 2014 Feb 10;7:1-9. doi: 10.4137/JCD.S13588. eCollection 2014. J Cell Death. 2014. PMID: 25278781 Free PMC article. Review. - Primary lysis of eosinophils as a major mode of activation of eosinophils in human diseased tissues.
Persson C, Uller L. Persson C, et al. Nat Rev Immunol. 2013 Dec;13(12):902. doi: 10.1038/nri3341-c1. Nat Rev Immunol. 2013. PMID: 24270781 No abstract available. - Pin1-FADD interactions regulate Fas-mediated apoptosis in activated eosinophils.
Oh J, Malter JS. Oh J, et al. J Immunol. 2013 May 15;190(10):4937-45. doi: 10.4049/jimmunol.1202646. Epub 2013 Apr 19. J Immunol. 2013. PMID: 23606538 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous