Normal and cystic fibrosis airway surface liquid homeostasis. The effects of phasic shear stress and viral infections - PubMed (original) (raw)
Normal and cystic fibrosis airway surface liquid homeostasis. The effects of phasic shear stress and viral infections
Robert Tarran et al. J Biol Chem. 2005.
Abstract
Mammalian airways normally regulate the volume of a thin liquid layer, the periciliary liquid (PCL), to facilitate the mucus clearance component of lung defense. Studies under standard (static) culture conditions revealed that normal airway epithelia possess an adenosine-regulated pathway that blends Na+ absorption and Cl- secretion to optimize PCL volume. In cystic fibrosis (CF), the absence of CF transmembrane conductance regulator results in a failure of adenosine regulation of PCL volume, which is predicted to initiate mucus stasis and infection. However, under conditions that mimic the phasic motion of the lung in vivo, ATP release into PCL was increased, CF ion transport was rebalanced, and PCL volume was restored to levels adequate for lung defense. This ATP signaling system was vulnerable, however, to insults that trigger CF bacterial infections, such as viral (respiratory syncytial virus) infections, which up-regulated extracellular ATPase activity and abolished motion-dependent ATP regulation of CF PCL height. These studies demonstrate (i) how the normal coordination of opposing ion transport pathways to maintain PCL volume is disrupted in CF, (ii) the hitherto unknown role of phasic motion in regulating key aspects of normal and CF innate airways defense, and (iii) that maneuvers directed at increasing motion-induced nucleotide release may be therapeutic in CF patients.
Figures
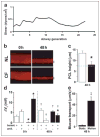
FIGURE 1. Abnormal regulation of PCL height by CF airway epithelia
a, XZ confocal images of PCL at 0, 6, and 48 h after mucosal addition of 20 _μ_l of PBS containing Texas-red dextran to NL and CF bronchial epithelial cultures. b, mean PCL height with time taken from NL (squares, n = 9) and CF (circles, n = 8) cultures. Note that the blue-shaded region denotes normal height of outstretched cilia (i.e. normal PCL height). c, electron micrographs of bronchial epithelial culture surfaces fixed with OsO4/PFC 48 h after PBS addition. d, bar graphs depicting changes in transepithelial electric potential difference (Vt) in response to bumetanide (10−4 M, basolateral) and amiloride (3 × 10−4 M, apical) in NL (open bars, n = 6) and CF (closed bars, n = 7) cultures at 0 and 48 h. All scale bars are 7 _μ_m. Data shown are the mean ± S.E. *, significantly different from t = 0. †, significantly different between NL and CF cultures.
FIGURE 2. Regulation of Na+ absorption/Cl− secretion by signals encoded in the PCL
a, xz confocal images of PCL immediately (0) and 6 and 48 h after mucosal addition of PBS with 5 units/ml apyrase to NL and CF bronchial epithelia. b, mean PCL height over 48 h for NL cultures exposed to apyrase (5 units/ml; closed squares, n = 9) or apyrase and 8-SPT (10−5 M; triangles, n = 5); CF cultures (n = 7) exposed to apyrase are denoted by red circles. c, change in Vt in response to bumetanide (bumet., 10−4 M, basolateral) and amiloride (amil., 3 × 10−4 M, apical) in NL (open bars, n = 8) and CF (closed bars, n = 4) cultures before (0 h) and 48 h after apyrase-exposure. d, mean PCL height after mucosal the addition of 8-SPT (10−5 M) alone at t = 0 to NL (squares, n = 7) and CF (circles, n = 6) cultures. Data shown as the mean ± S.E. †, data significantly different from t = 0. ‡, data significantly different between NL and CF cultures.
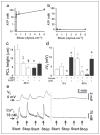
FIGURE 3. Phasic motion-induced changes in PCL volume and epithelial bioelectric properties in NL versus CF cultures
a, airflow induced in vivo shear stress as a function of airway generation at airway walls (see the supplemental material). The trachea is denoted by generation 0. b, xz confocal images of PCL immediately (0) and 48 h after mucosal PBS addition to NL and CF epithelia cultured under phasic motion. c, mean PCL heights after 48 h of phasic motion culture for NL (open bars, n = 7) and CF (closed bars, n = 8). d, bumetanide (bumet.) and amiloride (amil.)-sensitive Vt in NL (open bars, n = 7) and CF (closed bars, n = 4) cultures before (0) and after 48 h of phasic motion. e, rotational mucus transport rates in static CF cultures (closed bars, n = 12) and phasic motion cultures (gray bars, n = 14) 48 h after volume addition. Data are shown as mean ± S.E. *, data significantly different between NL and CF cultures. †, data significantly different from t = 0. ‡, significantly different between static and phasic motion.
FIGURE 4. Phasic motion-induced PCL secretion requires activation of Cl− channels
Mean PCL height after 3 h of phasic motion in the presence of a CFTR antagonist (CFTRinh172; 10 _μ_M) or CFTRinh172 and a CaCC antagonist (DIDS; 100 _μ_M). NLs (open bars; n = 6) and CFs (closed bars; n = 6). Data are shown as the mean ± S.E. *, data significantly different between NL and CF cultures. †, data significantly different from control. ‡, data significantly different from CFTRinh172.
FIGURE 5. Effects of phasic motion are mediated by ATP release into PCL
Mean PCL [ATP] (a) and mean serosal bath [ATP] (b) obtained from CF cultures 1 h after 50 μ_l of PBS addition under variable phasic motion (both, n = 4). NL PCL [ATP] was not significantly different from CF PCL ATP (1.9 ± 0.6, 12 ± 4, 95 ± 13, and 131 ± 41 nM at 0, 0.006, 0.6, and 6 dynes/cm2, respectively; all n = 4 and p < 0.05). c, mean PCL height after 48 h of phasic motion in the presence of mucosal apyrase (apyr., 5 units/ml) and/or 8-SPT (10 μ_M) in NL (open bars; n = 6 – 8) and CF (closed bars; n = 5–7). d, bumetanide (bumet.) and amiloride (amil.)-sensitive changes in Vt in NL (open bars, n = 8) and CF (closed bars, n = 4) cultures before (0) and 48 h after PBS addition/phasic motion in the presence of 5 units/ml mucosal apyrase. Note that amiloride and bumetanide values for CF cultures at 48 h are significantly different from the values without apyrase at 48 h (Fig. 3_d). e, simultaneous measurements of Vt and intracellular calcium (Ca2+i) in CF cultures perfused bilaterally with Ringer solution. Left, changes in Vt/Ca2+i induced by stopping then restarting mucosal perfusion (denoted by arrows). Right, altered perfusion rates in the presence of mucosal apyrase (5 units/ml). The mean changes in Vt and Ca2+i responses to phasic perfusion with KBR were −7.8 ± 0.3 mV and 198 ± 12 nM, respectively (p < 0.05; n = 7); the changes in each parameter were significantly reduced in the presence of apyrase; Δ_Vt = −0.2 ± 0.1 mV, ΔCa2+ = 3 ± 2 nM, respectively (p < 0.05; n = 6). Data shown as the mean ± S.E. *, data significantly different between NL and CF cultures. †, data significantly different from t = 0. ‡, data significantly different from apyrase. §, data significantly different from 8-SPT.
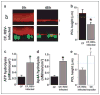
FIGURE 6. RSV infections inhibit nucleotide-dependent PCL homeostasis in CF cultures
a, XZ confocal images of PCL (red) covering control or RSV-_gfp_-infected ciliated cells (green) before (0) and 48 h after mucosal PBS addition to CF cultures under phasic motion conditions. b, PCL height 48 h after the addition of PBS for control CF cultures (closed bars; n = 10) and CF cultures infected with RSV-gfp (gray bars, n = 10). Also shown are mucosal ATP (c) and Mucosal Ap4A (d) hydrolysis rates in CF epithelia in control (closed bars) and RSV-infected cultures (gray bars). Both, n = 3. e, PCL height 48 h after the addition of PBS for CF cultures infected with RSV-gfp (gray bars, n = 5), and for cultures exposed to PCL and serosal media, but not virus, from RSV-infected cultures for 48 h under phasic motion conditions (CF transfer, open bars, n = 5). Data are shown as the mean ± S.E. *, difference (p < 0.05) between control and RSV-infected CF epithelia. †, difference (p < 0.05) between RSV-infected and CF-RSV media-exposed epithelia.
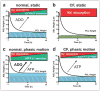
FIGURE 7. Schema describing PCL height regulation by active ion transport
a, NL airway epithelia under static conditions coordinately regulate the rates of Na+ absorption and Cl− secretion to adjust PCL volume from an “excessive” PCL height (25 _μ_m) to the physiologic PCL height with time. The blue color depicts PCL height as referenced to extended cilia. b, in CF epithelia, the higher basal rate of Na+ absorption, the failure to inhibit Na+ transport rates, and the failure to initiate Cl− secretion under static conditions lead to PCL depletion (note “flattened” cilia). c, NL airway epithelia under phasic motion respond to increased nucleotide/nucleoside release into PCL by shifting the balance toward Cl− secretion via CFTR (and CaCC; not shown) and a higher PCL. d, CF cultures under phasic motion conditions release sufficient ATP into the PCL to inhibit Na+ absorption and initiate CaCC-mediated Cl− secretion to restore PCL to a physiologically adequate height.
Similar articles
- Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia.
Tarran R, Trout L, Donaldson SH, Boucher RC. Tarran R, et al. J Gen Physiol. 2006 May;127(5):591-604. doi: 10.1085/jgp.200509468. J Gen Physiol. 2006. PMID: 16636206 Free PMC article. - Cystic fibrosis airway epithelial Ca2+ i signaling: the mechanism for the larger agonist-mediated Ca2+ i signals in human cystic fibrosis airway epithelia.
Ribeiro CM, Paradiso AM, Carew MA, Shears SB, Boucher RC. Ribeiro CM, et al. J Biol Chem. 2005 Mar 18;280(11):10202-9. doi: 10.1074/jbc.M410617200. Epub 2005 Jan 12. J Biol Chem. 2005. PMID: 15647273 - 17beta-Estradiol inhibits Ca2+-dependent homeostasis of airway surface liquid volume in human cystic fibrosis airway epithelia.
Coakley RD, Sun H, Clunes LA, Rasmussen JE, Stackhouse JR, Okada SF, Fricks I, Young SL, Tarran R. Coakley RD, et al. J Clin Invest. 2008 Dec;118(12):4025-35. doi: 10.1172/JCI33893. Epub 2008 Nov 20. J Clin Invest. 2008. PMID: 19033671 Free PMC article. - Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress.
Tarran R, Button B, Boucher RC. Tarran R, et al. Annu Rev Physiol. 2006;68:543-61. doi: 10.1146/annurev.physiol.68.072304.112754. Annu Rev Physiol. 2006. PMID: 16460283 Review. - Adenosine receptors, cystic fibrosis, and airway hydration.
Com G, Clancy JP. Com G, et al. Handb Exp Pharmacol. 2009;(193):363-81. doi: 10.1007/978-3-540-89615-9_12. Handb Exp Pharmacol. 2009. PMID: 19639288 Review.
Cited by
- ENaC regulation by proteases and shear stress.
Shi S, Carattino MD, Hughey RP, Kleyman TR. Shi S, et al. Curr Mol Pharmacol. 2013 Mar;6(1):28-34. doi: 10.2174/18744672112059990027. Curr Mol Pharmacol. 2013. PMID: 23547932 Free PMC article. Review. - Method for quantitative study of airway functional microanatomy using micro-optical coherence tomography.
Liu L, Chu KK, Houser GH, Diephuis BJ, Li Y, Wilsterman EJ, Shastry S, Dierksen G, Birket SE, Mazur M, Byan-Parker S, Grizzle WE, Sorscher EJ, Rowe SM, Tearney GJ. Liu L, et al. PLoS One. 2013;8(1):e54473. doi: 10.1371/journal.pone.0054473. Epub 2013 Jan 23. PLoS One. 2013. PMID: 23372732 Free PMC article. - Mucus-targeting therapies of defective mucus clearance for cystic fibrosis: A short review.
Figueira MF, Ribeiro CMP, Button B. Figueira MF, et al. Curr Opin Pharmacol. 2022 Aug;65:102248. doi: 10.1016/j.coph.2022.102248. Epub 2022 Jun 8. Curr Opin Pharmacol. 2022. PMID: 35689870 Free PMC article. Review. - Fluid flow induces mechanosensitive ATP release, calcium signalling and Cl- transport in biliary epithelial cells through a PKCzeta-dependent pathway.
Woo K, Dutta AK, Patel V, Kresge C, Feranchak AP. Woo K, et al. J Physiol. 2008 Jun 1;586(11):2779-98. doi: 10.1113/jphysiol.2008.153015. Epub 2008 Apr 3. J Physiol. 2008. PMID: 18388137 Free PMC article. - What's new in cystic fibrosis? From treating symptoms to correction of the basic defect.
Proesmans M, Vermeulen F, De Boeck K. Proesmans M, et al. Eur J Pediatr. 2008 Aug;167(8):839-49. doi: 10.1007/s00431-008-0693-2. Epub 2008 Apr 4. Eur J Pediatr. 2008. PMID: 18389279 Review.
References
- Guggino WB. Nat Med. 2001;7:888–889. - PubMed
- Verkman AS. Am J Physiol. 2001;281:L306–L308. - PubMed
- Puchelle E, de Bentzmann S, Zahm JM. Respiration. 1995;62(Suppl 1):2–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL60280/HL/NHLBI NIH HHS/United States
- P50 HL060280/HL/NHLBI NIH HHS/United States
- P01 HL034322/HL/NHLBI NIH HHS/United States
- R01 HL076303/HL/NHLBI NIH HHS/United States
- HL34322/HL/NHLBI NIH HHS/United States
- HL074158/HL/NHLBI NIH HHS/United States
- R01 HL074158/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases