An AP-1/clathrin coat plays a novel and essential role in forming the Weibel-Palade bodies of endothelial cells - PubMed (original) (raw)
An AP-1/clathrin coat plays a novel and essential role in forming the Weibel-Palade bodies of endothelial cells
Winnie W Y Lui-Roberts et al. J Cell Biol. 2005.
Abstract
Clathrin provides an external scaffold to form small 50-100-nm transport vesicles. In contrast, formation of much larger dense-cored secretory granules is driven by selective aggregation of internal cargo at the trans-Golgi network; the only known role of clathrin in dense-cored secretory granules formation is to remove missorted proteins by small, coated vesicles during maturation of these spherical organelles. The formation of Weibel-Palade bodies (WPBs) is also cargo driven, but these are cigar-shaped organelles up to 5 mum long. We hypothesized that a cytoplasmic coat might be required to make these very different structures, and we found that new and forming WPBs are extensively, sometimes completely, coated. Overexpression of an AP-180 truncation mutant that prevents clathrin coat formation or reduced AP-1 expression by small interfering RNA both block WPB formation. We propose that, in contrast to other secretory granules, cargo aggregation alone is not sufficient to form immature WPBs and that an external scaffold that contains AP-1 and clathrin is essential.
Figures
Figure 1.
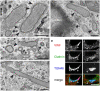
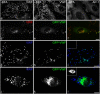
Perinuclear WPBs are coated. (a–d) Conventional transmission EM of HUVECs shows extensive coating of WPBs. Bars, 200 nm. (a) Arrowheads point to the coat covering a WPB. (b) Such coating is more often observed in the perinuclear region (arrowheads). (c) A transverse section of a WPB (arrowhead) and a typical CCV (arrow) have different diameters. (d) Partial coating (arrowhead) on a WPB is seen where the VWF tubules are less structured. (e) Newly emerging WPBs at the TGN (arrowheads) in two different cells partially colocalize with clathrin by immunofluorescence. Bar, 10 μm.
Figure 2.
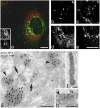
AP-1 is present on WPBs. (a–e) Partial colocalization of AP-1 and VWF shown by immunofluorescence. HUVECs were fixed in 6% PFA, permeabilized, and labeled with mouse anti–AP-1 and rabbit anti-VWF, followed by FITC-conjugated anti–mouse and Texas red–conjugated anti–rabbit secondary antibodies. Bars, 10 μm. (a) A perinuclear WPB is substantially covered with AP-1. The insets show a higher magnification of the boxed area (top inset, AP-1; bottom inset, VWF). (b–e) Further examples of partial colocalization of VWF (b and d) and AP-1 (c and e). (f–h) Immuno-EM of HUVEC cryosections show the presence of AP-1 on WPBs. Bars, 200 nm. (f) A WPB labeled with rabbit anti-VWF followed by 15 nm gold particles conjugated to protein A. (g) AP-1 is observed on CCVs (arrows) and it is also found at the rim of transverse sections of WPBs (arrowheads) near the Golgi. (h) An electron-dense coat that is labeled with an AP-1 antibody (arrowhead) is associated with an elongated VWF-containing structure.
Figure 3.
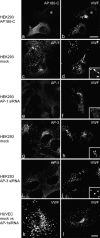
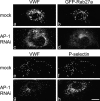
Clathrin and AP-1 are required for WPB biogenesis. (a and b) HEK293 cells were cotransfected with AP180-C and full-length VWF constructs. WPB formation was impaired in a cell transfected with AP180-C. In contrast, elongated WPBs were clearly seen in a cell that did not contain AP180-C (*). (c–j) The depletion of AP-1, but not AP-3, impairs the formation of WPBs in HEK293 cells. Cells were transfected with a full-length VWF construct and AP-1 siRNA (e and f), AP-3 siRNA (i and j), or mock transfected (c, d, g, and h). Although elongated WPBs were clearly observed upon AP-3 RNAi (j), WPBs formation was severely impaired upon AP-1 siRNA treatment (f). (k and l) Similar results were obtained in HUVECs. Elongated WPBs are abundant in a mock-treated cell (k) but not in the AP-1–depleted cell (l). Insets show a magnified view of the boxed regions. Bars, 10 μm.
Figure 4.
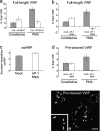
AP180-C and AP-1 RNAi cause a significant reduction in PMA-stimulated VWF secretion, which is not the result from the loss of TGN integrity nor from furin missorting. (a) HEK293 cells were cotransfected with a full-length VWF construct and AP180-C (white bars) or a control vector (gray bars). VWF secretion assays were performed 2–3 d after transfection. The PMA-responsive pool of VWF was estimated by subtracting the amount of VWF released from constitutive secretion from that released upon PMA addition. All data were normalized using the total signal of VWF (see Materials and methods). Each bar represents the mean ± SD, n = 3. (b and c) HEK293 cells were transfected with an siRNA targeted at the μ1 subunit of the AP-1 complex. After 3 d, the cells were further transfected with the same siRNA together with a full-length VWF construct (b) or an ssHRP construct (c). Secretion assays were performed 2–3 d after the second transfection. Although stimulated secretion of VWF was abolished, constitutive secretion of transfected ssHRP was not impaired in AP-1–depleted cells, suggesting the integrity of the TGN. The gray bars represent data from mock transfection, whereas the white bars represent data from the AP-1 RNAi. Each bar represents the mean ± SD, n = 4. *, see section Clathrin and AP-1 are essential for WPB biogenesis. (d) Cotransfection of the propeptide and mature VWF constructs failed to rescue the defect in regulated secretion in AP-1–depleted cells. Each bar represents the mean ± SD, n = 5. (e) Cotransfection with two VWF constructs containing the pro-region and the mature region, respectively, generated WPBs in control HEK293 cells. The cells were labeled with rabbit anti-VWF, followed by Texas red–conjugated donkey anti–rabbit secondary antibodies. Inset shows the magnified view of the boxed region. Bar, 10 μm.
Figure 5.
The AP-1/clathrin coat is involved in an early stage of WPB biogenesis, but not in the maintenance of the cigar shape of preformed WPBs. (a and b) A BFA-sensitive coat is not required for the maintenance of the shape of existing WPBs. HUVECs were treated with 5 μg/ml BFA for 30 min (b). Compared with untreated cells in panel a, there was no significant change to the appearance in anti-VWF staining. (c) Anti–γ-adaptin staining shows that AP-1 dissociates from WPBs and other membranes upon BFA treatment. (d–f) HUVECs were treated with 10 μM of monensin for 1 h (see Fig. S2), nucleofected with GFP-VWF, and allowed to recover overnight. The cells were labeled with rabbit anti-VWF (d), followed by Texas red–conjugated anti–rabbit antibody. Although the old rounded WPBs did not return to the original shape, newly synthesized WPBs had a classic rod shape (e). (g–l) HUVECs were microinjected with GFP-VWF together with a control vector plasmid (g–i) or AP180-C (j–l), fixed 16 h later, and labeled with anti-VWF (g and j; blue in i and l) and anti-GFP (h and k; green in i and l). Although old WPBs remained clearly visible in both cases, the cell microinjected with AP180-C failed to make new cigar-shaped WPBs. (insets) Anti-myc labeling shows the microinjected AP180-C construct. Bars, 10 μm.
Figure 6.
VWF-positive puncta in AP-1–depleted HUVECs cannot recruit WPB components. (a–d) GFP-Rab27a was recruited onto peripheral WPBs in mock-transfected cells (b), but upon AP-1 RNAi, became cytosolic (d) and failed to associate with the VWF-positive puncta (c). (e–h) HUVECs were treated with 100 μM leupeptin and 100 μM pepstatin for 6 h before fixation. The colocalization of P-selectin and VWF (e and f) was lost upon AP-1 RNAi (g and h). P-selectin was observed only after the lysosomal inhibitor treatment (h), suggesting that it was targeted to lysosomes for degradation in the absence of WPBs in AP-1–depleted cells. Bar, 10 μm.
Similar articles
- Discs large 1 (Dlg1) scaffolding protein participates with clathrin and adaptator protein complex 1 (AP-1) in forming Weibel-Palade bodies of endothelial cells.
Philippe M, Léger T, Desvaux R, Walch L. Philippe M, et al. J Biol Chem. 2013 May 3;288(18):13046-56. doi: 10.1074/jbc.M112.441261. Epub 2013 Mar 26. J Biol Chem. 2013. PMID: 23532850 Free PMC article. - Real-time imaging of the dynamics and secretory behavior of Weibel-Palade bodies.
Romani de Wit T, Rondaij MG, Hordijk PL, Voorberg J, van Mourik JA. Romani de Wit T, et al. Arterioscler Thromb Vasc Biol. 2003 May 1;23(5):755-61. doi: 10.1161/01.ATV.0000069847.72001.E8. Epub 2003 Apr 3. Arterioscler Thromb Vasc Biol. 2003. PMID: 12676800 - Aftiphilin and gamma-synergin are required for secretagogue sensitivity of Weibel-Palade bodies in endothelial cells.
Lui-Roberts WW, Ferraro F, Nightingale TD, Cutler DF. Lui-Roberts WW, et al. Mol Biol Cell. 2008 Dec;19(12):5072-81. doi: 10.1091/mbc.e08-03-0301. Epub 2008 Sep 24. Mol Biol Cell. 2008. PMID: 18815278 Free PMC article. - Biogenesis and exocytosis of Weibel-Palade bodies.
van Mourik JA, Romani de Wit T, Voorberg J. van Mourik JA, et al. Histochem Cell Biol. 2002 Feb;117(2):113-22. doi: 10.1007/s00418-001-0368-9. Epub 2002 Jan 19. Histochem Cell Biol. 2002. PMID: 11935287 Review. - How to roll an endothelial cigar: the biogenesis of Weibel-Palade bodies.
Michaux G, Cutler DF. Michaux G, et al. Traffic. 2004 Feb;5(2):69-78. doi: 10.1111/j.1600-0854.2004.00157.x. Traffic. 2004. PMID: 14690496 Review.
Cited by
- A defined clathrin-mediated trafficking pathway regulates sFLT1/VEGFR1 secretion from endothelial cells.
Kinghorn K, Gill A, Marvin A, Li R, Quigley K, Singh S, Gore MT, le Noble F, Gabhann FM, Bautch VL. Kinghorn K, et al. Angiogenesis. 2024 Feb;27(1):67-89. doi: 10.1007/s10456-023-09893-6. Epub 2023 Sep 11. Angiogenesis. 2024. PMID: 37695358 Free PMC article. - AP-1A controls secretory granule biogenesis and trafficking of membrane secretory granule proteins.
Bonnemaison M, Bäck N, Lin Y, Bonifacino JS, Mains R, Eipper B. Bonnemaison M, et al. Traffic. 2014 Oct;15(10):1099-121. doi: 10.1111/tra.12194. Epub 2014 Aug 15. Traffic. 2014. PMID: 25040637 Free PMC article. - Storage and regulated secretion of factor VIII in blood outgrowth endothelial cells.
van den Biggelaar M, Bouwens EA, Kootstra NA, Hebbel RP, Voorberg J, Mertens K. van den Biggelaar M, et al. Haematologica. 2009 May;94(5):670-8. doi: 10.3324/haematol.13427. Epub 2009 Mar 31. Haematologica. 2009. PMID: 19336741 Free PMC article. - Type II PI4-kinases control Weibel-Palade body biogenesis and von Willebrand factor structure in human endothelial cells.
Lopes da Silva M, O'Connor MN, Kriston-Vizi J, White IJ, Al-Shawi R, Simons JP, Mössinger J, Haucke V, Cutler DF. Lopes da Silva M, et al. J Cell Sci. 2016 May 15;129(10):2096-105. doi: 10.1242/jcs.187864. Epub 2016 Apr 11. J Cell Sci. 2016. PMID: 27068535 Free PMC article. - Widespread dysregulation of peptide hormone release in mice lacking adaptor protein AP-3.
Sirkis DW, Edwards RH, Asensio CS. Sirkis DW, et al. PLoS Genet. 2013;9(9):e1003812. doi: 10.1371/journal.pgen.1003812. Epub 2013 Sep 26. PLoS Genet. 2013. PMID: 24086151 Free PMC article.
References
- Arribas, M., and D.F. Cutler. 2000. Weibel-Palade body membrane proteins exhibit differential trafficking after exocytosis in endothelial cells. Traffic. 1:783–793. - PubMed
- Bonfanti, R., B.C. Furie, B. Furie, and D.D. Wagner. 1989. PADGEM (GMP140) is a component of Weibel-Palade bodies of human endothelial cells. Blood. 73:1109–1112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources