Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction - PubMed (original) (raw)
Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction
Nadine Wong Shi Kam et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Biological systems are known to be highly transparent to 700- to 1,100-nm near-infrared (NIR) light. It is shown here that the strong optical absorbance of single-walled carbon nanotubes (SWNTs) in this special spectral window, an intrinsic property of SWNTs, can be used for optical stimulation of nanotubes inside living cells to afford multifunctional nanotube biological transporters. For oligonucleotides transported inside living cells by nanotubes, the oligos can translocate into cell nucleus upon endosomal rupture triggered by NIR laser pulses. Continuous NIR radiation can cause cell death because of excessive local heating of SWNT in vitro. Selective cancer cell destruction can be achieved by functionalization of SWNT with a folate moiety, selective internalization of SWNTs inside cells labeled with folate receptor tumor markers, and NIR-triggered cell death, without harming receptor-free normal cells. Thus, the transporting capabilities of carbon nanotubes combined with suitable functionalization chemistry and their intrinsic optical properties can lead to new classes of novel nanomaterials for drug delivery and cancer therapy.
Figures
Fig. 1.
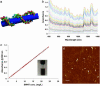
Carbon nanotubes with high NIR absorbance solubilized in water. (a) Schematic of a Cy3-DNA-functionalized SWNT. The drawing is only a graphic presentation and does not represent the precise way DNA binds on SWNTs. (b) UV-visible spectra of solutions of individual SWNTs functionalized noncovalently by 15-mer Cy3 labeled-DNA at various nanotube concentrations (top curve, SWNT concentration ≈25 mg/liter in H2O; lower curves correspond to consecutive 3% reduction in SWNT concentration). The well defined peaks in the UV-visible spectra suggest lack of large aggregated SWNTs in the solution by removing bundles by centrifugation. (c) Absorbance at 808 nm vs. SWNT concentration (optical path = 1 cm). Solid line is Beer's law fit to obtain molar extinction coefficient of SWNT ε ≈7.9 × 106 M–1·cm–1. (Inset) A photo of a DNA-functionalized SWNT solution. (d) AFM image of DNA-functionalized individual SWNTs (height of 1–10 nm) deposited on a SiO2 substrate. (Scale bar: 200 nm.)
Fig. 2.
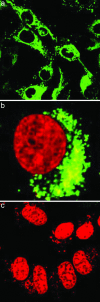
Transporting DNA inside living cells by SWNTs. (a) A confocal fluorescence image (excitation λ = 548 nm; emission detected at λ = 560 nm) showing the internalization and accumulation of Cy3-DNA-SWNT around the nucleus (circular regions surrounded by green fluorescence corresponding to Cy3) of HeLa cells after incubation of cells (≈4 × 105 cells per well in 12-well plates) for 12 h at 37°C in a 2.5–5 mg/liter Cy3-DNA-SWNT solution. (b) Dual detection of Cy3-DNA-SWNT (green) internalized into a HeLa cell with the nucleus stained by DRAQ5 (red). (c) A confocal image of HeLa cells after incubation in a Cy3-DNA-SWNT solution at a low temperature of 4°C. Only DRAQ5-stained nucleus (red color) of HeLa is seen. The lack of green fluorescence detected indicates that there is minimal cellular uptake of the Cy3-DNA-SWNT conjugates at the low temperature. (Magnification: ×63.)
Fig. 3.
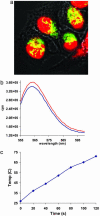
In vitro NIR excitation of SWNT transporters for DNA cargo releasing and nuclear translocation. (a) A confocal image (×63) of HeLa cells after 12-h incubation in a 2.5–5 mg/liter Cy3-DNA-SWNT solution for internalization and radiation by six NIR (808 nm) 10-s pulses (at 1.4 W/cm2 power density). Colocalization (yellow color) of Cy3-DNA (green) in cell nucleus (red) was detected, indicating translocation of Cy3-DNA to the nucleus. After incubation, the cells were washed and resuspended in cell medium in a quartz cuvette for NIR radiation (laser beam diameter ≈3 cm, power 10 W, optical path 1 cm). (b) Cy3 fluorescence emission spectra of a Cy3-DNA-SWNT solution (25 mg/liter) before (blue curve) and after (red curve) laser radiation (1.4 W/cm2) for 2 min. λexcitation = 550 nm and λemission = 563 nm. (c) An ex vitro control experiment. Temperature evolution of a DNA-SWNT solution (≈25 mg/liter) during continuous radiation by a 808-nm laser at 1.4 W/cm2 for 2 min. This result clearly reveals heating of solution caused by absorption of 808-nm laser light by SWNTs in the solution.
Fig. 4.
Fate of cells with internalized SWNTs after NIR laser pulses or extensive radiations. (a) Optical image of HeLa cells after DNA-SWNT uptake and six pulses of 10-s-, 808-nm laser radiations at 1.4 W/cm2. Cells retained normal morphology with no apparent death observed. (Inset) Photo of the cell solution taken 12 h after laser pulses. Pink color was caused by phenol red in the well. HeLa cells remained adhered to the bottom of the container. (b) Image of HeLa cells without internalized SWNTs after continuous 808-nm laser radiation at 3.5 W/cm2 for 5 min. No cell death was observed. (Inset) Photo of cell solution after radiation. (c) Image of dead and aggregated cells after internalization of DNA-SWNT and laser radiation at 1.4 W/cm2 for 2 min. The dead cells showed rounded and aggregated morphology as opposed to live cells in a “stretched” form in a and b. (Inset) Photo of the cell solution 24 h after laser-activated cell death. No live cells adherent to the bottom of the container were observed. Black aggregates containing SWNTs released from dead cells floating on water were visible (indicated by arrow) to the naked eye. (d) Raman data and scanning electron microscopy image (Inset) of the black aggregates after drying. (Magnifications: ×20 for confocal images and ×36,000 for scanning electron microscopy.)
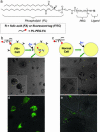
Fig. 5.
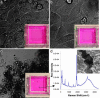
Selective targeting and killing of cancer cells. (a) Chemical structure of PL-PEG-FA and PL-PEG-FITC synthesized by conjugating PL-PEG-NH2 with FA or FITC, respectively, for solubilizing individual SWNTs. (b) (Upper) Schematic of selective internalization of PL-PEG-FA-SWNTs into folate-overexpressing (FR+) cells via receptor binding and then NIR 808-nm laser radiation. (Lower) Image showing death of FR+ cells with rounded cell morphology after the process in Upper (808-nm laser radiation at 1.4 W/cm2 for 2 min). (Inset) Higher-magnification image shows details of the killed cells. (c) (Upper) Schematic of no internalization of PL-PEG-FA-SWNTs into normal cells without available FRs. (Lower) Image showing normal cells with no internalized SWNTs are unharmed by the same laser radiation condition as in b. (Inset) Higher magnification image shows a live normal cell in stretched shape. (d) Confocal image of FR+ cells after incubation in a solution of SWNTs with two cargoes (PL-PEG-FA and PL-PEG-FITC). The strong green FITC fluorescence inside cells confirms the SWNT uptake with FA and FITC cargoes. (e) The same as d for normal cells without abundant FRs on cell surfaces. There is little green fluorescence inside cells, confirming little uptake of SWNTs with FA and FITC cargoes. (Magnifications: ×20.)
Similar articles
- Cancer photothermal therapy in the near-infrared region by using single-walled carbon nanotubes.
Zhou F, Xing D, Ou Z, Wu B, Resasco DE, Chen WR. Zhou F, et al. J Biomed Opt. 2009 Mar-Apr;14(2):021009. doi: 10.1117/1.3078803. J Biomed Opt. 2009. PMID: 19405722 Free PMC article. - In vivo near-infrared mediated tumor destruction by photothermal effect of carbon nanotubes.
Moon HK, Lee SH, Choi HC. Moon HK, et al. ACS Nano. 2009 Nov 24;3(11):3707-13. doi: 10.1021/nn900904h. ACS Nano. 2009. PMID: 19877694 - Anti-HER2 IgY antibody-functionalized single-walled carbon nanotubes for detection and selective destruction of breast cancer cells.
Xiao Y, Gao X, Taratula O, Treado S, Urbas A, Holbrook RD, Cavicchi RE, Avedisian CT, Mitra S, Savla R, Wagner PD, Srivastava S, He H. Xiao Y, et al. BMC Cancer. 2009 Oct 2;9:351. doi: 10.1186/1471-2407-9-351. BMC Cancer. 2009. PMID: 19799784 Free PMC article. - Carbon nanotubes for biomedical imaging: the recent advances.
Gong H, Peng R, Liu Z. Gong H, et al. Adv Drug Deliv Rev. 2013 Dec;65(15):1951-63. doi: 10.1016/j.addr.2013.10.002. Epub 2013 Oct 30. Adv Drug Deliv Rev. 2013. PMID: 24184130 Review. - Single-walled carbon nanotubes as near-infrared optical biosensors for life sciences and biomedicine.
Jain A, Homayoun A, Bannister CW, Yum K. Jain A, et al. Biotechnol J. 2015 Mar;10(3):447-59. doi: 10.1002/biot.201400168. Epub 2015 Feb 13. Biotechnol J. 2015. PMID: 25676253 Review.
Cited by
- 3D viability imaging of tumor phantoms treated with single-walled carbon nanohorns and photothermal therapy.
Whitney J, DeWitt M, Whited BM, Carswell W, Simon A, Rylander CG, Rylander MN. Whitney J, et al. Nanotechnology. 2013 Jul 12;24(27):275102. doi: 10.1088/0957-4484/24/27/275102. Epub 2013 Jun 18. Nanotechnology. 2013. PMID: 23780336 Free PMC article. - A chitosan-modified graphene nanogel for noninvasive controlled drug release.
Wang C, Mallela J, Garapati US, Ravi S, Chinnasamy V, Girard Y, Howell M, Mohapatra S. Wang C, et al. Nanomedicine. 2013 Oct;9(7):903-11. doi: 10.1016/j.nano.2013.01.003. Epub 2013 Jan 22. Nanomedicine. 2013. PMID: 23352802 Free PMC article. - Nanoplatforms for magnetic resonance imaging of cancer.
Cywińska MA, Grudziński IP, Cieszanowski A, Bystrzejewski M, Popławska M. Cywińska MA, et al. Pol J Radiol. 2011 Apr;76(2):28-36. Pol J Radiol. 2011. PMID: 22802828 Free PMC article. - Tumor targeting and imaging in live animals with functionalized semiconductor quantum rods.
Yong KT, Hu R, Roy I, Ding H, Vathy LA, Bergey EJ, Mizuma M, Maitra A, Prasad PN. Yong KT, et al. ACS Appl Mater Interfaces. 2009 Mar;1(3):710-9. doi: 10.1021/am8002318. ACS Appl Mater Interfaces. 2009. PMID: 20160901 Free PMC article. - Conductive and Thermo-Responsive Composite Hydrogels with Poly(N-isopropylacrylamide) and Carbon Nanotubes Fabricated by Two-Step Photopolymerization.
Ciarleglio G, Toto E, Santonicola MG. Ciarleglio G, et al. Polymers (Basel). 2023 Feb 18;15(4):1022. doi: 10.3390/polym15041022. Polymers (Basel). 2023. PMID: 36850305 Free PMC article.
References
- Henry, C. M. (2004) Chem. Eng. News 82, 37–42.
- Alivisatos, P. (2004) Nat. Biotechnol. 22, 47–52. - PubMed
- Taton, T., Mirkin, C. & Letsinger, R. (2000) Science 289, 1757–1760. - PubMed
- Cui, Y., Wei, Q., Park, H. & Lieber, C. (2001) Science 293, 1289–1292. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous