Olig2 directs astrocyte and oligodendrocyte formation in postnatal subventricular zone cells - PubMed (original) (raw)
Olig2 directs astrocyte and oligodendrocyte formation in postnatal subventricular zone cells
Christine A G Marshall et al. J Neurosci. 2005.
Abstract
The subventricular zone (SVZ) in the neonatal mammalian forebrain simultaneously generates olfactory interneurons, astrocytes, and oligodendrocytes. The molecular cues that enable SVZ progenitors to generate three distinct cell lineages without a temporal switching mechanism are not known. Here, we demonstrate that the basic helix-loop-helix transcription factor Olig2 plays a central role in this process. Olig2 is specifically expressed in gliogenic progenitors in the postnatal SVZ and by all glial lineages derived from this structure. By expressing normal and dominant-interfering forms of Olig2 in vivo, we show that Olig2 repressor function is both sufficient and necessary to prevent neuronal differentiation and to direct SVZ progenitors toward astrocytic and oligodendrocytic fates. Although Olig2 activity has been associated previously with motor neuron and oligodendrocyte development, our findings establish a previously unappreciated role for Olig2 in the development of astrocytes. Furthermore, these results indicate that Olig2 serves a critical role in pan-glial versus neuronal fate decisions in SVZ progenitors, making it the first intrinsic fate determinant shown to operate in the early postnatal SVZ.
Figures
Figure 1.
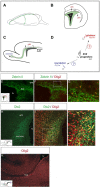
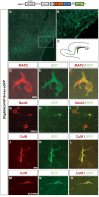
Olig2 expression in the SVZ. A, Diagram of coronal and parasagittal planes highlighting the SVZ (B) and RMS (C) (both green), respectively. SVZ-derived glial precursors migrate radially within a coronal plane into adjacent forebrain areas, whereas SVZ-derived neuronal progenitors migrate rostrally in a parasagittal plane to the olfactory bulb. D, Transcriptional regulators directing the formation of neuronal and glial lineages in SVZ cells are not known. E-G, Olig2 expression in Zebrin II+ (border) and Zebrin II- (middle) SVZ cells, as indicated by immunostaining of P6 mouse forebrain. The inset in E depicts the area of photomicrographs shown in E and F. The boxed area in F is magnified in G. H-J, Olig2 is expressed in Dlx2+ (central) SVZ progenitors. The inset in H depicts the area of the photomicrograph shown in H and I. The boxed area in I is magnified in J. K, Cells within the RMS do not express Olig2. lv, Lateral ventricle; wm, white matter.
Figure 2.
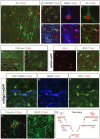
Developing astrocytes and oligodendrocytes express Olig2. A, Cortical radial glia with an intermediate morphology (arrowheads), indicative of a transformation into astrocytes, express Zebrin II and Olig2 (P3, coronal section through murine cerebral cortex). B-D, Olig2 and Zebrin II are expressed robustly in developing astrocytes (P6, murine cerebral cortex) at early stages of differentiation before GFAP expression (B, arrowheads) and at later stages as GFAP is upregulated (B, asterisk). The cell marked with an asterisk in B is magnified to show perinuclear GFAP (C) and Zebrin II (D) immunolabeling with respect to nuclear Olig2 expression. E-G, Mature and fully arborized astrocytes (P6, murine cerebral cortex) expressing high amounts of GFAP and Zebrin II (E, arrowheads) consistently appeared to express lower levels of Olig2 than astrocytes with immature morphologies that express less GFAP (E, asterisk). H, I, CNPase+/Olig2+ oligodendrocytes in parasagittal sections of P6 mouse forebrain. J, K, Developing astrocytes in the hGFAP-GFP mouse (P7) forebrain coexpress GFP and Olig2. L, M, Astrocytes in the mOlig2-I-eGFP mouse forebrain (P6, cortex) coexpress GFAP, GFP, and Olig2. O, Cell cultures generated from P3 mouse SVZ contained many vimentin+/Olig2+, immature astrocytes after 4 div. P, After 8 div, cultures were mostly comprised of mature, GFAP+ astrocytes. Olig2 immunoreactivity in these mature astrocytes was markedly reduced. Q, Summary of Olig2 expression with respect to glial cell lineages in the postnatal forebrain.
Figure 3.
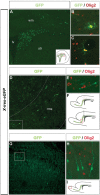
Olig2 is expressed exclusively by SVZ-derived glia. A, GFP expression 2 d after stereotactic injection of replication-deficient retrovirus (pNIT-eGFP) into P2/P3 rat SVZ. See F for the site of injection and distribution of infected cells at 4 dpi. B, C, At 4 dpi, GFP+/Olig2+ cells with oligodendrocyte (B) and astrocyte (C) morphologies were observed in the parenchyma (the asterisk in C indicates a blood vessel enwrapped by an astrocyte). D, E, SVZ-derived neuroblasts do not express Olig2 as they migrate in the RMS, which is outlined for clarity. The boxed area is magnified in E. F, Distribution of SVZ cells transduced with GFP. The red box indicates the area of the photomicrograph in D. G-I, SVZ-derived interneuronal precursors remain Olig2-within the olfactory bulb. The boxed area in G is magnified in H. I, The red box indicates the area of the photomicrograph in G. lv, Lateral ventricle; wm, white matter; str, striatum.
Figure 4.
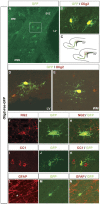
SVZ progenitors transduced with Olig2 differentiate exclusively into astrocytes and oligodendrocytes. A-C, Parasagittal section of rat forebrain at 4 dpi, showing cells transduced with Olig2-ires-eGFP. The boxed area in A is magnified in B, highlighting differentiated glial cells within the SVZ/RMS. C, Distribution of cells expressing Olig2-ires-eGFP at 4 dpi. Thered box indicates the area of SVZ and proximal RMS shown in A. D, Infected cells differentiated ectopically in the SVZ, adjacent to the lateral ventricles. E-K, More than 70% of infected cells exhibited the morphology of oligodendrocytes (E) and expressed the oligodendrocyte-specific markers NG2 (F-H) and CC1 (I-K). L-N, Approximately 20% of infected cells exhibited astrocyte morphologies and expressed GFAP (asterisk). lv, Lateral ventricle; wm, white matter; str, striatum.
Figure 5.
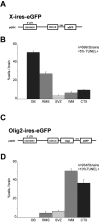
Distribution of SVZ cells expressing X-ires-eGFP and Olig2-ires-eGFP. A-D, Retroviral constructs used to transduce SVZ cells with GFP (A) or Olig2-GFP (C) and quantified distributions of cells misexpressing GFP (B) or Olig2-ires-GFP (D), presented as a percentage of infected cells per brain located in each area (mean ± SEM). OB, Olfactory bulb; WM, white matter; CTX, cerebral cortex.
Figure 6.
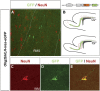
A dominant-interfering form of Olig2 blocks glial differentiation. A, B, SVZ cells misexpressing Olig2 bHLH exhibited normal migration to the olfactory bulb via the RMS (A), which is outlined for clarity. The distribution of total infected cells at 4 dpi is shown in B. The red box indicates the area of RMS pictured (A). Infected cells also colonized the white matter and cerebral cortex, but glial differentiation was blocked in >90% of cells (data not shown). C-E, However, ∼25% of infected cells in the white matter and cortex ectopically expressed the neuronal marker NeuN, suggesting a potential respecification along a neuronal lineage. wm, White matter.
Figure 7.
An antimorphic activator form of Olig2 promotes neurogenesis in SVZ cells. A-C, Expression of an Olig2bHLH-VP16 activator fusion construct limited the migration of SVZ cells. Cells migrated short distances into the proximal RMS, white matter, and layer 6 of the cerebral cortex. The boxed area in A, containing infected SVZ cells, is magnified in B. The distribution of infected cells is shown in C. D-I, More than 75% of infected cells expressed the neuronal markers Map2 (D-F) and NeuN (G-I). J-O, Approximately 30% of cells in the white matter and SVZ/RMS expressed interneuron-specific markers such as calbindin (J-L) and calretinin (M-O). WM/VI, White matter/layer 6 border; LV, lateral ventricle.
Similar articles
- Regional- and temporal-dependent changes in the differentiation of Olig2 progenitors in the forebrain, and the impact on astrocyte development in the dorsal pallium.
Ono K, Takebayashi H, Ikeda K, Furusho M, Nishizawa T, Watanabe K, Ikenaka K. Ono K, et al. Dev Biol. 2008 Aug 15;320(2):456-68. doi: 10.1016/j.ydbio.2008.06.001. Epub 2008 Jun 11. Dev Biol. 2008. PMID: 18582453 - Origin of oligodendrocytes in the subventricular zone of the adult brain.
Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Menn B, et al. J Neurosci. 2006 Jul 26;26(30):7907-18. doi: 10.1523/JNEUROSCI.1299-06.2006. J Neurosci. 2006. PMID: 16870736 Free PMC article. - Dlx1 and Dlx2 control neuronal versus oligodendroglial cell fate acquisition in the developing forebrain.
Petryniak MA, Potter GB, Rowitch DH, Rubenstein JL. Petryniak MA, et al. Neuron. 2007 Aug 2;55(3):417-33. doi: 10.1016/j.neuron.2007.06.036. Neuron. 2007. PMID: 17678855 Free PMC article. - Olig2 transcription factor in the developing and injured forebrain; cell lineage and glial development.
Ono K, Takebayashi H, Ikenaka K. Ono K, et al. Mol Cells. 2009 Apr 30;27(4):397-401. doi: 10.1007/s10059-009-0067-2. Epub 2009 Apr 13. Mol Cells. 2009. PMID: 19390819 Review. - Lineage, migration, and fate determination of postnatal subventricular zone cells in the mammalian CNS.
Goldman JE. Goldman JE. J Neurooncol. 1995;24(1):61-4. doi: 10.1007/BF01052660. J Neurooncol. 1995. PMID: 8523077 Review.
Cited by
- A conserved molecular logic for neurogenesis to gliogenesis switch in the cerebral cortex.
Liang XG, Hoang K, Meyerink BL, Kc P, Paraiso K, Wang L, Jones IR, Zhang Y, Katzman S, Finn TS, Tsyporin J, Qu F, Chen Z, Visel A, Kriegstein A, Shen Y, Pilaz LJ, Chen B. Liang XG, et al. Proc Natl Acad Sci U S A. 2024 May 14;121(20):e2321711121. doi: 10.1073/pnas.2321711121. Epub 2024 May 7. Proc Natl Acad Sci U S A. 2024. PMID: 38713624 Free PMC article. - 5Z-7-Oxozaenol attenuates cuprizone-induced demyelination in mice through microglia polarization regulation.
Chen S, Liu S, Huang Y, Huang S, Zhang W, Xie H, Lu L. Chen S, et al. Brain Behav. 2024 Apr;14(4):e3487. doi: 10.1002/brb3.3487. Brain Behav. 2024. PMID: 38648385 Free PMC article. - Abundant transcriptomic alterations in the human cerebellum of patients with a C9orf72 repeat expansion.
Udine E, DeJesus-Hernandez M, Tian S, das Neves SP, Crook R, Finch NA, Baker MC, Pottier C, Graff-Radford NR, Boeve BF, Petersen RC, Knopman DS, Josephs KA, Oskarsson B, Da Mesquita S, Petrucelli L, Gendron TF, Dickson DW, Rademakers R, van Blitterswijk M. Udine E, et al. Acta Neuropathol. 2024 Apr 19;147(1):73. doi: 10.1007/s00401-024-02720-2. Acta Neuropathol. 2024. PMID: 38641715 Free PMC article. - Dynamic Changes in Neuroglial Reaction and Tissue Repair after Photothrombotic Stroke in Neonatal Mouse.
Liu Y, Gong P, Qi G, Tang H, Gui R, Qi C, Qin S. Liu Y, et al. Brain Sci. 2024 Feb 1;14(2):152. doi: 10.3390/brainsci14020152. Brain Sci. 2024. PMID: 38391727 Free PMC article. - Single-cell genomics reveals region-specific developmental trajectories underlying neuronal diversity in the human hypothalamus.
Herb BR, Glover HJ, Bhaduri A, Colantuoni C, Bale TL, Siletti K, Hodge R, Lein E, Kriegstein AR, Doege CA, Ament SA. Herb BR, et al. Sci Adv. 2023 Nov 10;9(45):eadf6251. doi: 10.1126/sciadv.adf6251. Epub 2023 Nov 8. Sci Adv. 2023. PMID: 37939194 Free PMC article.
References
- Altman J (1966) Proliferation and migration of undifferentiated precursor cells in the rat during postnatal gliogenesis. Exp Neurol 16: 263-278. - PubMed
- Altman J (1969) Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol 137: 433-458. - PubMed
- Anthony TE, Klein C, Fishell G, Heinz N (2004) Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron 41: 881-890. - PubMed
- Bertrand N, Castro DS, Guillemot F (2002) Proneural genes and the specification of neural cell types. Nat Neurosci Rev 3: 517-530. - PubMed
- Briscoe J, Ericson J (2001) Specification of neuronal fates in the ventral neural tube. Curr Opin Neurobiol 11: 43-49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources