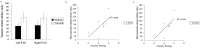A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging - PubMed (original) (raw)
Comparative Study
A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging
Paul Dunckley et al. J Neurosci. 2005.
Abstract
Evidence from both human and animal studies has demonstrated a key role for brainstem centers in the control of ascending nociceptive input. Nuclei such as the rostral ventromedial medulla and periaqueductal gray (PAG) are able to both inhibit and facilitate the nociceptive response. It has been proposed that altered descending modulation may underlie many of the chronic pain syndromes (both somatic and visceral). We used functional magnetic resonance imaging to image the neural correlates of visceral and somatic pain within the brainstem. Ten healthy subjects were scanned twice at 3 tesla, during which they received matched, moderately painful, electrical stimuli to either the midline lower abdomen or rectum. Significant activation was observed in regions consistent with the PAG, nucleus cuneiformis (NCF), ventral tegmental area/substantia nigra, parabrachial nuclei/nucleus ceruleus, and red nucleus bilaterally to both stimuli. Marked spatial similarities in activation were observed for visceral and somatic pain, although significantly greater activation of the NCF (left NCF, p = 0.02; right NCF, p = 0.01; Student's paired t test, two-tailed) was observed in the visceral pain group compared with the somatic group. Right PAG activity correlated with anxiety during visceral stimulation (r = 0.74; p < 0.05, Pearson's r, two-tailed) but not somatic stimulation. We propose that the differences in NCF and right PAG activation observed may represent a greater nocifensive response and greater emotive salience of visceral over somatic pain.
Figures
Figure 1.
Graphical representation of the slice orientation during the functional scan. For clarity, the underlying image is a T1-weighted structural scan, and the number of slices was reduced from 24 to 8. A coronal-oblique orientation was used with slices aligned with the ventral wall of the fourth ventricle.
Figure 2.
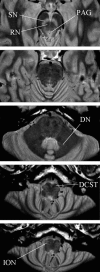
Axial slices through a PDTSE sequence structural scan of the brainstem. These were designed to provide maximal resolution and contrast within the brainstem to aid accurate region of interest mask formation. The substantia nigra (SN) and PAG are seen in lighter contrast. The red nucleus (RN), dentate nucleus (DN), decussation of the corticospinal tracts (DCST), and inferior olivary nucleus (ION) are labeled.
Figure 3.
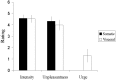
The group psychophysical data. The stimuli were well matched, with no significant differences in either intensity or unpleasantness rating between visceral or somatic stimulation (p > 0.05; Student's t test, paired, two-tailed). Electrical stimulation of the rectum induced a mild urge sensation. Error bars represent SE.
Figure 4.
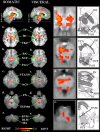
Group activation maps for somatic (first column) and visceral (second column) pain. The sagittal slices show columns of activation within the brainstem in each group. Axial slices (a-e) correspond to the plane indicated in the sagittal slice. A reduced field of view was used during functional scanning; thus, the activation map is limited in its anteroposterior plane. The two groups have a similar spatial pattern of activation. Regions commonly activated in whole-brain pain-imaging studies are significantly activated: thalamus (Thal) and posterior insula (Ins) bilaterally. Activation was also seen in the globus pallidus (GP) bilaterally in both groups. Significantly, activated brainstem nuclei included the red nucleus (RN), NCF, PAG, VTA [which extended laterally into the substantia nigra (SN)], and the dorsolateral pons (DLPons) bilaterally. Bilateral activation of the PN occurred in the visceral group but was limited to the right side in the somatic group. Activation in the region of the RVM was also seen in both groups. In the visceral group alone, a region of activation occurred in the left dorsolateral medulla (DLM). A small area of motion artifact (MA) occurred around the central canal in the somatic group. The axial slices in the visceral group have been magnified and juxtaposed to drawings at the same anatomical location [modified from Duvernoy (1995) with permission].
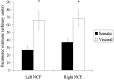
Figure 5.
Nucleus cuneiformis activity. Significantly greater activation occurred bilaterally in the NCF during visceral pain (*p < 0.05; Student's t test, two-tailed, paired, uncorrected). Error bars represent SE.
Figure 6.
Correlation of anxiety with PAG activity during visceral pain. a, The mean PAG parameter estimate for visceral and somatic pain; no significant difference in activation was observed. a, b, Anxiety during the painful visceral stimuli correlated with right (R) PAG activity (b; r = 0.74; p < 0.05; Pearson's r, two-tailed) and approached significance for the left (L) PAG (a; r = 0.66; p = 0.08; Pearson's r, two-tailed).
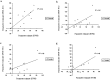
Figure 7.
Correlation analysis between RVM activity and that of the dorsolateral pons in the region of the PBN and NC (PBN-NC). A significant correlation was seen bilaterally for visceral stimulation (a, b) (right, r = 0.65, p = 0.04; left, r = 0.75, p = 0.01; Pearson's r, two-tailed) but not for somatic stimulation (c, d) (right, r = 0.51, p = 0.13; left, r = 0.75, p = 0.03; Pearson's r, two-tailed). R, Right; L, left.
Similar articles
- Cortical processing of visceral and somatic stimulation: differentiating pain intensity from unpleasantness.
Dunckley P, Wise RG, Aziz Q, Painter D, Brooks J, Tracey I, Chang L. Dunckley P, et al. Neuroscience. 2005;133(2):533-42. doi: 10.1016/j.neuroscience.2005.02.041. Neuroscience. 2005. PMID: 15896917 - Anticipatory brainstem activity predicts neural processing of pain in humans.
Fairhurst M, Wiech K, Dunckley P, Tracey I. Fairhurst M, et al. Pain. 2007 Mar;128(1-2):101-10. doi: 10.1016/j.pain.2006.09.001. Epub 2006 Oct 27. Pain. 2007. PMID: 17070996 - Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome.
Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Ohning G, Kilpatrick L, Bueller JA, Ruby K, Jarcho J, Mayer EA. Berman SM, et al. J Neurosci. 2008 Jan 9;28(2):349-59. doi: 10.1523/JNEUROSCI.2500-07.2008. J Neurosci. 2008. PMID: 18184777 Free PMC article. Clinical Trial. - Visceral pain.
Raj PP. Raj PP. Agri. 2004 Jan;16(1):7-20. Agri. 2004. PMID: 15152583 Review. - Brain responses to visceral and somatic stimuli in irritable bowel syndrome: a central nervous system disorder?
Chang L. Chang L. Gastroenterol Clin North Am. 2005 Jun;34(2):271-9. doi: 10.1016/j.gtc.2005.02.003. Gastroenterol Clin North Am. 2005. PMID: 15862935 Review.
Cited by
- Stress-induced visceral pain: toward animal models of irritable-bowel syndrome and associated comorbidities.
Moloney RD, O'Mahony SM, Dinan TG, Cryan JF. Moloney RD, et al. Front Psychiatry. 2015 Feb 16;6:15. doi: 10.3389/fpsyt.2015.00015. eCollection 2015. Front Psychiatry. 2015. PMID: 25762939 Free PMC article. Review. - Functional brain imaging of gastrointestinal sensation in health and disease.
Van Oudenhove L, Coen SJ, Aziz Q. Van Oudenhove L, et al. World J Gastroenterol. 2007 Jul 7;13(25):3438-45. doi: 10.3748/wjg.v13.i25.3438. World J Gastroenterol. 2007. PMID: 17659690 Free PMC article. Review. - Pain management mini-series. Part I. A review of management of acute pain.
Johnson Q, Borsheski RR, Reeves-Viets JL. Johnson Q, et al. Mo Med. 2013 Jan-Feb;110(1):74-9. Mo Med. 2013. PMID: 23457757 Free PMC article. Review. - Orofacial pain of cardiac origin, serial of clinical cases.
López-López J, Adserias-Garriga MJ, Garcia-Vicente L, Jané-Salas E, Chimenos-Küstner E, Pereferrer-Kleiner D. López-López J, et al. Med Oral Patol Oral Cir Bucal. 2012 Jul 1;17(4):e633-7. doi: 10.4317/medoral.17689. Med Oral Patol Oral Cir Bucal. 2012. PMID: 22322498 Free PMC article.
References
- Aicher SA, Randich A (1990) Antinociception and cardiovascular responses produced by electrical stimulation in the nucleus tractus solitarius, nucleus reticularis ventralis, and the caudal medulla. Pain 42: 103-119. - PubMed
- Almy T, Kern F, Tulin M (1949) Alteration in colonic function in man under stress. II. Experimental production of sigmoid spasm in healthy persons. Gastroenterology 12: 425-436. - PubMed
- Andrew LK, Blackshaw LA (2001) Colonic mechanoreceptor inputs to rat lumbo-sacral dorsal horn neurones: distribution, thresholds and chemosensory modulation. Neurogastroenterol Motil 13: 333-337. - PubMed
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I (2002) Imaging how attention modulates pain in humans using functional MRI. Brain 125: 310-319. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical