Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis - PubMed (original) (raw)
Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis
Robyn S Klein et al. J Virol. 2005 Sep.
Abstract
The activation and entry of antigen-specific CD8(+) T cells into the central nervous system is an essential step towards clearance of West Nile virus (WNV) from infected neurons. The molecular signals responsible for the directed migration of virus-specific T cells and their cellular sources are presently unknown. Here we demonstrate that in response to WNV infection, neurons secrete the chemokine CXCL10, which recruits effector T cells via the chemokine receptor CXCR3. Neutralization or a genetic deficiency of CXCL10 leads to a decrease in CXCR3(+) CD8(+) T-cell trafficking, an increase in viral burden in the brain, and enhanced morbidity and mortality. These data support a new paradigm in chemokine neurobiology, as neurons are not generally considered to generate antiviral immune responses, and CXCL10 may represent a novel neuroprotective agent in response to WNV infection in the central nervous system.
Figures
FIG. 1.
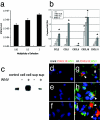
Quantitative PCR analyses of chemokine, cytokine and chemokine receptor expression in WNV-infected brain tissues. a. Eight-week-old C57BL/6 mice were inoculated with 102 PFU of WNV by footpad injection and sacrificed on day 8 postinfection. Brains were recovered after cardiac perfusion with phosphate-buffered saline and titrated for virus by plaque assay. Shown are total RNA prepared from whole brains of three mice analyzed for the expression of T-cell chemoattractants and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) via quantitative PCR. Plaque assay data are expressed as 0 (below limit of detection, open bars), 2 × 104 (gray bars) and 5 × 105 (black bars) PFU and chemokine data are expressed as copies per copy of glyceraldehyde-3-phosphate dehydrogenase. b to g. Five-week-old C57BL/6 mice were similarly infected and sacrificed in groups of 5 on days 2, 5, 8, and 10 postinfection. Total RNA prepared from frontal cortices and cerebella were analyzed for the expression ofCCL2, CCL5, CCL7, CXCL9, and CXCL11 (b to c); CXCL10 (d); gamma interferon and tumor necrosis factor alpha (e and f); and CCR1, CCR2, CCR5, and CXCR3 (g) quantitative PCR. Average values are from a representative of two experiments done in quintuplicate and presented as ± standard error of the mean. Significance of chemokine, cytokine, and chemokine receptor expression in WNV-infected animals was determined by comparison with uninfected animals (day 0).
FIG. 2.
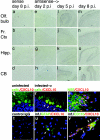
Cellular localization of CXCL10 expression in WNV-infected CNS. In situ hybridization analyses of brain sections from WNV-infected mice 2 (e to h), 5 (i to l), and 8 (a to d and m to p) days postinfection using digoxigenin-labeled sense (a to d) and antisense (e to p) riboprobes. Immunohistochemical analyses of CXCL10 (red) expression within Calbindin (green), neuron-specific enolase (green), glial fibrillary acidic protein (green), and CD11b (green)-expressing cells in brain sections from uninfected (q) and WNV-infected (r to v) mice 8 days postinfection. Nuclei have been counterstained with DAPI. Arrowheads: colocalization (yellow) of CXCL10 and Calbindin (r), neuron-specific enolase (s), glial fibrillary acidic protein (u), and CD11b (v) within neurons (r and s), occasional astrocytes (u), and infiltrating macrophages associated with a vessel (v). All original magnifications, ×100 (a to p) and ×400 (q to v); analyses were performed on sections from eight animals, n = 2 mice per group. Abbreviations: Olf. Bulb, olfactory bulb; Fr. Ct, frontal cortex; Hipp., hippocampus; CB, cerebellum; NSE, neuron-specific enolase; GFAP, glial fibrillary acidic protein.
FIG. 3.
Colocalization of WNV and CXCL10 within neurons in vivo. Immunohistochemical analyses of WNV antigen (brown) and CXCL10 (blue) within neurons of brain sections from uninfected (a and b) and WNV-infected (c and d) RAG1 T- and B-cell-deficient congenic C57BL/6 mice. Red arrowheads: colocalization of brown and blue chromogen within neurons of the frontal cortex (c) and CA2 region of hippocampal formation (d). All magnifications, ×400.
FIG. 4.
WNV-infected neurons express chemokines, especially CXCL10. Primary cultures of cerebellar granule cell neurons were prepared as described in Materials and Methods, infected with WNV at a multiplicity of infection of 0.03, 0.3, or 3 and evaluated for production of infectious WNV via plaque assay on Vero cells (a) and total RNA prepared from WNV-infected and uninfected cultures was evaluated for chemokine mRNA expression via quantitative PCR (b). Data derived from two separate experiments with duplicates or triplicates and expressed as mean ± standard error of the mean. Western blot analysis of CXCL10 protein expression in supernatants (sup) and cell pellets (cell) of WNV-infected and uninfected granule cell neurons compared with 125 pg of recombinant CXCL10 (control) (c) and double label immunofluorescence microscopy for expression of CXCL10 protein (red) and WNV antigen (green) within uninfected (d to f) and WNV-infected (g to i) granule cell (d and g), cortical (e and h) and hippocampal (f and i) neurons. Nuclei have been counterstained with DAPI. Arrows: colocalization (yellow) of CXCL10 protein and WNV antigen. All magnifications, ×400.
FIG. 5.
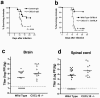
Survival data of wild-type and CXCL10-deficient mice. Eight-week-old C57BL/6 mice were administered anti-CXCL10 or an isotype control intraperitoneally on days 0, 2, 4, and 6 postinfection with 102 PFU of WNV via footpad and then followed for 28 days (a). The survival curves were constructed using data from three independent experiments with n = 15 for each antibody treatment. Survival differences between isotype- and CXCL10 antibody-treated mice were statistically significant (P = 0.036). Eight week-old wild-type and congenic CXCL10-deficient C57BL/6 mice were inoculated via footpad with 102 PFU of WNV and followed for 28 days (b). The survival curves were constructed using data from two independent experiments with n = 12 for each group. Survival differences between wild-type and CXCL10-deficient mice were statistically significant (P = 0.0001). Viral loads in the CNS after WNV infection. WNV burden in the (c) brain and (d) spinal cord of wild-type and CXCL10-deficient mice at day 10 after infection. Virus levels were measured using a plaque assay after tissues were harvested at day 10. Data are shown as the average PFU per gram of tissue and reflect 16 to 20 wild-type mice and 12 CXCL10-deficient mice. The dotted line represents the limit of sensitivity of the assay. The differences in viral burden between the wild-type and CXCL10-deficient mice were statistically significant as judged by comparison of the medians using the Mann-Whitney test for brain (P < 0.05) and spinal cord (P < 0.03).
FIG. 6.
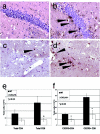
T-cell trafficking into the brains of WNV-infected CXCL10-deficient mice is impaired. Immunohistochemical analyses of CD3+ cells (brown) within hippocampi (a and b) and midbrain (c and d) from 5-week-old WNV-infected CXCL10-deficient (a and c) and wild-type (b and d) mice at 8 days postinfection. All sections have been counterstained with hematoxylin; black arrowheads indicate individual CD3+ cells and cell clusters. All magnifications, ×200. Data are representative of staining experiments performed on four mice. Flow cytometric analyses of total CD4+ and CD8+ T cells (e) and CXCR3-expressing CD4+ and CD8+ T cells (f) derived from pooled brains and spinal cords of 5-week-old wild-type (solid bars) and CXCL10-deficient (open bars) mice. Average values of CD4+ and CD8+ cells are presented as total cell numbers per three pooled CNS tissues from four experiments in which there were six or nine mice per genotype infected with WNV ± standard error of the mean.
Similar articles
- CXCR3 mediates region-specific antiviral T cell trafficking within the central nervous system during West Nile virus encephalitis.
Zhang B, Chan YK, Lu B, Diamond MS, Klein RS. Zhang B, et al. J Immunol. 2008 Feb 15;180(4):2641-9. doi: 10.4049/jimmunol.180.4.2641. J Immunol. 2008. PMID: 18250476 - CD40-CD40 ligand interactions promote trafficking of CD8+ T cells into the brain and protection against West Nile virus encephalitis.
Sitati E, McCandless EE, Klein RS, Diamond MS. Sitati E, et al. J Virol. 2007 Sep;81(18):9801-11. doi: 10.1128/JVI.00941-07. Epub 2007 Jul 11. J Virol. 2007. PMID: 17626103 Free PMC article. - TNF-alpha-dependent regulation of CXCR3 expression modulates neuronal survival during West Nile virus encephalitis.
Zhang B, Patel J, Croyle M, Diamond MS, Klein RS. Zhang B, et al. J Neuroimmunol. 2010 Jul 27;224(1-2):28-38. doi: 10.1016/j.jneuroim.2010.05.003. Epub 2010 Jun 25. J Neuroimmunol. 2010. PMID: 20579746 Free PMC article. - Immunology of West Nile Virus Infection and the Role of Alpha-Synuclein as a Viral Restriction Factor.
Lesteberg KE, Beckham JD. Lesteberg KE, et al. Viral Immunol. 2019 Jan/Feb;32(1):38-47. doi: 10.1089/vim.2018.0075. Epub 2018 Sep 15. Viral Immunol. 2019. PMID: 30222521 Review. - Innate and adaptive immune responses determine protection against disseminated infection by West Nile encephalitis virus.
Diamond MS, Shrestha B, Mehlhop E, Sitati E, Engle M. Diamond MS, et al. Viral Immunol. 2003;16(3):259-78. doi: 10.1089/088282403322396082. Viral Immunol. 2003. PMID: 14583143 Review.
Cited by
- Versatile tissue-injectable hydrogels capable of the extended hydrolytic release of bioactive protein therapeutics.
Nealy ES, Reed SJ, Adelmund SM, Badeau BA, Shadish JA, Girard EJ, Brasel K, Pakiam FJ, Mhyre AJ, Price JP, Sarkar S, Kalia V, DeForest CA, Olson JM. Nealy ES, et al. Bioeng Transl Med. 2024 Apr 15;9(5):e10668. doi: 10.1002/btm2.10668. eCollection 2024 Sep. Bioeng Transl Med. 2024. PMID: 39553428 Free PMC article. - T Cells Trafficking into the Brain in Aging and Alzheimer's Disease.
Ma YZ, Cao JX, Zhang YS, Su XM, Jing YH, Gao LP. Ma YZ, et al. J Neuroimmune Pharmacol. 2024 Aug 24;19(1):47. doi: 10.1007/s11481-024-10147-5. J Neuroimmune Pharmacol. 2024. PMID: 39180590 Review. - Transcriptomic analysis unveils bona fide molecular signatures of microglia under conditions of homeostasis and viral encephalitis.
Mulenge F, Gern OL, Busker LM, Aringo A, Ghita L, Waltl I, Pavlou A, Kalinke U. Mulenge F, et al. J Neuroinflammation. 2024 Aug 17;21(1):203. doi: 10.1186/s12974-024-03197-2. J Neuroinflammation. 2024. PMID: 39153993 Free PMC article. - Orchestration of antiviral responses within the infected central nervous system.
Pavlou A, Mulenge F, Gern OL, Busker LM, Greimel E, Waltl I, Kalinke U. Pavlou A, et al. Cell Mol Immunol. 2024 Sep;21(9):943-958. doi: 10.1038/s41423-024-01181-7. Epub 2024 Jul 12. Cell Mol Immunol. 2024. PMID: 38997413 Free PMC article. Review. - Impact of double-strand breaks induced by uv radiation on neuroinflammation and neurodegenerative disorders.
Vijayakumar S, Yesudhason BV, Anandharaj JL, Sathyaraj WV, Selvan Christyraj JRS. Vijayakumar S, et al. Mol Biol Rep. 2024 Jun 8;51(1):725. doi: 10.1007/s11033-024-09693-1. Mol Biol Rep. 2024. PMID: 38851636 Review.
References
- Asensio, V. C., C. Kincaid, and I. L. Campbell. 1999. Chemokines and the inflammatory response to viral infection in the central nervous system with a focus on lymphocytic choriomeningitis virus. J. Neurovirol. 5:65-75. - PubMed
- Asensio, V. C., J. Maier, R. Milner, K. Boztug, C. Kincaid, M. Moulard, C. Phillipson, K. Lindsley, T. Krucker, H. S. Fox, and I. L. Campbell. 2001. Interferon-independent, human immunodeficiency virus type 1 gp120-mediated induction of CXCL10/IP-10 gene expression by astrocytes in vivo and in vitro. J. Virol. 75:7067-7077. - PMC - PubMed
- Brewer, G. J. 1995. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J. Neurosci Res. 42:674-683. - PubMed
- Chen, C. J., S. L. Liao, M. D. Kuo, and Y. M. Wang. 2000. Astrocytic alteration induced by Japanese encephalitis virus infection. Neuroreport 11:1933-1937. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01NS052632-01/NS/NINDS NIH HHS/United States
- K02NS045607/NS/NINDS NIH HHS/United States
- K02 NS045607/NS/NINDS NIH HHS/United States
- R01 CA069212/CA/NCI NIH HHS/United States
- R01 NS052632/NS/NINDS NIH HHS/United States
- R01CA069212/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials