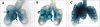Fog2 is required for normal diaphragm and lung development in mice and humans - PubMed (original) (raw)
doi: 10.1371/journal.pgen.0010010. Epub 2005 Jun 17.
Bruce J Herron, Sara O Vargas, Hailu Huang, Sergei G Tevosian, Lazaros Kochilas, Cherie Rao, Barbara R Pober, Randal P Babiuk, Jonathan A Epstein, John J Greer, David R Beier
Affiliations
- PMID: 16103912
- PMCID: PMC1183529
- DOI: 10.1371/journal.pgen.0010010
Fog2 is required for normal diaphragm and lung development in mice and humans
Kate G Ackerman et al. PLoS Genet. 2005 Jul.
Abstract
Congenital diaphragmatic hernia and other congenital diaphragmatic defects are associated with significant mortality and morbidity in neonates; however, the molecular basis of these developmental anomalies is unknown. In an analysis of E18.5 embryos derived from mice treated with N-ethyl-N-nitrosourea, we identified a mutation that causes pulmonary hypoplasia and abnormal diaphragmatic development. Fog2 (Zfpm2) maps within the recombinant interval carrying the N-ethyl-N-nitrosourea-induced mutation, and DNA sequencing of Fog2 identified a mutation in a splice donor site that generates an abnormal transcript encoding a truncated protein. Human autopsy cases with diaphragmatic defect and pulmonary hypoplasia were evaluated for mutations in FOG2. Sequence analysis revealed a de novo mutation resulting in a premature stop codon in a child who died on the first day of life secondary to severe bilateral pulmonary hypoplasia and an abnormally muscularized diaphragm. Using a phenotype-driven approach, we have established that Fog2 is required for normal diaphragm and lung development, a role that has not been previously appreciated. FOG2 is the first gene implicated in the pathogenesis of nonsyndromic human congenital diaphragmatic defects, and its necessity for pulmonary development validates the hypothesis that neonates with congenital diaphragmatic hernia may also have primary pulmonary developmental abnormalities.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
Figure 1. Abnormal Pulmonary and Diaphragmatic Development in the lil Mouse
(A) The mutant hypoplastic lung (right) lacks the development of the accessory lobe and the anterior portion of the right middle lobe (marked with arrows on the control sample on the left). (B) Whole diaphragms show a lack of normal muscularization in the posterolateral regions and the peripheral regions of the mutant diaphragm (control on left and lil diaphragm on the right).
Figure 2. The ENU-Induced lil Mutation in Fog2 is a Splice Site Mutation
(A) RT-PCR revealed a lengthened transcript in mice with the lil phenotype: the first band is a lil mouse, and the second band is a control mouse. (B) Sequencing Fog2 revealed a splice site mutation that causes the insertion of 85 bp of intronic sequence in the mutant mouse. This results in a premature stop codon prior to zinc finger transcription.
Figure 3. Fog2 Is Expressed in the Developing Lung and Diaphragm
Fog2 is expressed in the diffuse pulmonary mesenchyme at E13.5 (A) (arrow shows mesenchyme) and is restricted to the bronchial and vascular smooth muscle (sm) at E16.5 (B). Fog2 is expressed diffusely in the developing diaphragm (Dia) both prior to (E11.5) (C) and after muscularization (E13.5) (D).
Figure 4. Fog2 Is Necessary for Primary Lung Development
Fog2 null lungs removed prior to diaphragmatic muscularization and grown in vitro show no accessory lobe development. Accessory lobe is labeled with black arrow in control littermate lungs.
Figure 5. Fog2 Expression in Embryonic Non-Mutant Lungs
In embryonic non-mutant lungs, Fog2 is most highly expressed at the tips of the accessory (single arrows) and right middle lobes (double arrows) at E11.25 (A) and E11.75 (B), while expression is diffuse by E12.75 (C).
Figure 6. HGF Patterning Is Abnormal in Fog2 Null Mice
In situ hybridization of HGF in E12.5 wild-type (A) and Fog2 −/− (B) embryos demonstrates decreased expression in the region where the PPF meets the membranous diaphragm. Li, liver; Lu, lung.
Figure 7. FOG2 Mutation in a Patient with Diaphragm and Lung Abnormalities
Sequencing revealed a de novo heterozygote nonsense mutation in a patient who died at birth with severe pulmonary hypoplasia and a posterior deep diaphragmatic eventration. She was clinically diagnosed with CDH. This nonsense mutation occurs prior to the functional zinc finger domains.
Similar articles
- Candidate genes for congenital diaphragmatic hernia from animal models: sequencing of FOG2 and PDGFRalpha reveals rare variants in diaphragmatic hernia patients.
Bleyl SB, Moshrefi A, Shaw GM, Saijoh Y, Schoenwolf GC, Pennacchio LA, Slavotinek AM. Bleyl SB, et al. Eur J Hum Genet. 2007 Sep;15(9):950-8. doi: 10.1038/sj.ejhg.5201872. Epub 2007 Jun 13. Eur J Hum Genet. 2007. PMID: 17568391 - Prenatal retinoic acid up-regulates pulmonary gene expression of COUP-TFII, FOG2, and GATA4 in pulmonary hypoplasia.
Doi T, Sugimoto K, Puri P. Doi T, et al. J Pediatr Surg. 2009 Oct;44(10):1933-7. doi: 10.1016/j.jpedsurg.2009.04.027. J Pediatr Surg. 2009. PMID: 19853750 - Impaired mesenchymal cell function in Gata4 mutant mice leads to diaphragmatic hernias and primary lung defects.
Jay PY, Bielinska M, Erlich JM, Mannisto S, Pu WT, Heikinheimo M, Wilson DB. Jay PY, et al. Dev Biol. 2007 Jan 15;301(2):602-14. doi: 10.1016/j.ydbio.2006.09.050. Epub 2006 Oct 5. Dev Biol. 2007. PMID: 17069789 Free PMC article. - Abnormal lung development in congenital diaphragmatic hernia.
Ameis D, Khoshgoo N, Keijzer R. Ameis D, et al. Semin Pediatr Surg. 2017 Jun;26(3):123-128. doi: 10.1053/j.sempedsurg.2017.04.011. Epub 2017 Apr 25. Semin Pediatr Surg. 2017. PMID: 28641748 Review. - Review of the phenotypic spectrum associated with haploinsufficiency of MYRF.
Rossetti LZ, Glinton K, Yuan B, Liu P, Pillai N, Mizerik E, Magoulas P, Rosenfeld JA, Karaviti L, Sutton VR, Lalani SR, Scott DA. Rossetti LZ, et al. Am J Med Genet A. 2019 Jul;179(7):1376-1382. doi: 10.1002/ajmg.a.61182. Epub 2019 May 8. Am J Med Genet A. 2019. PMID: 31069960 Free PMC article. Review.
Cited by
- Role of genetics and the environment in the etiology of congenital diaphragmatic hernia.
Liu S, Yu L. Liu S, et al. World J Pediatr Surg. 2024 Aug 21;7(3):e000884. doi: 10.1136/wjps-2024-000884. eCollection 2024. World J Pediatr Surg. 2024. PMID: 39183805 Free PMC article. Review. - FOXP1 Haploinsufficiency Contributes to the Development of Congenital Diaphragmatic Hernia.
Pendleton KE, Hernandez-Garcia A, Lyu JM, Campbell IM, Shaw CA, Vogt J, High FA, Donahoe PK, Chung WK, Scott DA. Pendleton KE, et al. J Pediatr Genet. 2023 Mar 28;13(1):29-34. doi: 10.1055/s-0043-1767731. eCollection 2024 Mar. J Pediatr Genet. 2023. PMID: 38567173 Free PMC article. - A Genomic Link From Heart Failure to Atrial Fibrillation Risk: FOG2 Modulates a TBX5/GATA4-Dependent Atrial Gene Regulatory Network.
Broman MT, Nadadur RD, Perez-Cervantes C, Burnicka-Turek O, Lazarevic S, Gams A, Laforest B, Steimle JD, Iddir S, Wang Z, Smith L, Mazurek SR, Olivey HE, Zhou P, Gadek M, Shen KM, Khan Z, Theisen JWM, Yang XH, Ikegami K, Efimov IR, Pu WT, Weber CR, McNally EM, Svensson EC, Moskowitz IP. Broman MT, et al. Circulation. 2024 Apr 9;149(15):1205-1230. doi: 10.1161/CIRCULATIONAHA.123.066804. Epub 2024 Jan 8. Circulation. 2024. PMID: 38189150 - Radiomics-Assisted Computed Tomography-Based Analysis to Evaluate Lung Morphology Characteristics after Congenital Diaphragmatic Hernia.
Virlan SV, Froelich MF, Thater G, Rafat N, Elrod J, Boettcher M, Schoenberg SO, Weis M. Virlan SV, et al. J Clin Med. 2023 Dec 15;12(24):7700. doi: 10.3390/jcm12247700. J Clin Med. 2023. PMID: 38137769 Free PMC article. - The etiology of congenital diaphragmatic hernia: the retinoid hypothesis 20 years later.
Rivas JFG, Clugston RD. Rivas JFG, et al. Pediatr Res. 2024 Mar;95(4):912-921. doi: 10.1038/s41390-023-02905-7. Epub 2023 Nov 21. Pediatr Res. 2024. PMID: 37990078 Free PMC article. Review.
References
- Langham MR, Jr, Kays DW, Ledbetter DJ, Frentzen B, Sanford LL, et al. Congenital diaphragmatic hernia. Epidemiology and outcome. Clin Perinatol. 1996;23:671–688. - PubMed
- Downard CD, Jaksic T, Garza JJ, Dzakovic A, Nemes L, et al. Analysis of an improved survival rate for congenital diaphragmatic hernia. J Pediatr Surg. 2003;38:729–732. - PubMed
- Javid PJ, Jaksic T, Skarsgard ED, Lee S. Survival rate in congenital diaphragmatic hernia: The experience of the Canadian Neonatal Network. J Pediatr Surg. 2004;39:657–660. - PubMed
- Stege G, Fenton A, Jaffray B. Nihilism in the 1990s: The true mortality of congenital diaphragmatic hernia. Pediatrics. 2003;112:532–535. - PubMed
- Harrington KP, Goldman AP. The role of extracorporeal membrane oxygenation in congenital diaphragmatic hernia. Semin Pediatr Surg. 2005;14:72–76. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases