Molecular mechanism of temperature sensing by the circadian clock of Neurospora crassa - PubMed (original) (raw)
. 2005 Sep 1;19(17):1968-73.
doi: 10.1101/gad.345905. Epub 2005 Aug 17.
Affiliations
- PMID: 16107616
- PMCID: PMC1199567
- DOI: 10.1101/gad.345905
Molecular mechanism of temperature sensing by the circadian clock of Neurospora crassa
Axel C R Diernfellner et al. Genes Dev. 2005.
Abstract
Expression levels and ratios of the long (l) and short (s) isoforms of the Neurospora circadian clock protein FREQUENCY (FRQ) are crucial for temperature compensation of circadian rhythms. We show that the ratio of l-FRQ versus s-FRQ is regulated by thermosensitive splicing of intron 6 of frq, a process removing the translation initiation site of l-FRQ. Thermosensitivity is due to inefficient recognition of nonconsensus splice sites at elevated temperature. The temperature-dependent accumulation of FRQ relative to bulk protein is controlled at the level of translation. The 5'-UTR of frq RNA contains six upstream open reading frames (uORFs) that are in nonconsensus context for translation initiation. Thermosensitive trapping of scanning ribosomes at the uORFs leads to reduced translation of the main ORF and allows adjustment of FRQ levels according to ambient temperature.
Figures
Figure 1.
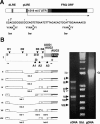
The 5′-UTR of frq RNA is spliced in a complex fashion. (A, top) The frq gene is schematically outlined. Distal light-responsive elements (dLRE) and proximal light-responsive elements (pLRE) are indicated by hatched boxes (Froehlich et al. 2002). The major transcription initiation site is indicated by an arrow. The black and the gray + black areas correspond to s-FRQ and l-FRQ, respectively. (Bottom) Major and minor transcription initiation sites. Eleven independent clones of cap-specific RLM-RACE products were sequenced. Numbers above the sequence indicate the 5′-ends of these clones. The major initiation site (seven clones) corresponds to position -1519. Initiator consensus sequences (Inr) for transcription (Smail and Kadonga 2003) are indicated below the sequence. (Y indicates C or T; N indicates any nucleotide). (B, top) Schematic outline of the 5′-UTR of frq. Splice donor (D) and acceptor (A) sites and translation initiation sites for l-FRQ (AUG1) and s-FRQ (AUG3) are shown. AUG2 is not used for initiation (Liu et al. 1997). Dark-gray arrows indicate uORF1 to uORF6 in the 5′-UTR and ORF7 overlapping the AUG of s-FRQ. The arrows below the scheme indicate oligonucleotide primers for amplification of cDNA. The outlined unspliced (I) and spliced (II-VIII) frq RNA species (left panel) were amplified by reverse transcription and PCR (right panel) and identified by sequencing (black arrowheads) or by diagnostic PCR (white arrowheads). RNA was prepared from cells grown at 25°C in LL. (Std) DNA size standard; (gDNA) genomic DNA.
Figure 2.
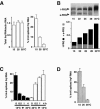
I-6 is spliced in a temperature-dependent fashion. (A) Neurospora was grown at the indicated temperatures in LL, and RNA was prepared and quantitfied by RT-PCR (see Materials and Methods). (Left) Total frq RNA was determined. frq RNA/actin RNA at 35°C was set equal to 1. (Right) The fraction (%) of frq RNA spliced at I-6 (spliced/total frq RNA) was determined. (B) Protein extracts prepared from Neurospora that was grown at the indicated temperatures in LL were treated with alkaline phosphatase. Samples were analyzed by immunoblotting with αFRQ, recognizing l-FRQ and s-FRQ (top) and quantified by densitometry (bottom). l-FRQ (black bars) and s-FRQ (gray bars) are shown. (C) Cultures were shifted from 15°C to 35°C (dark bars) or from 35°C to 15°C (white bars). The fraction of spliced I-6 was determined. (D) Thermosensitive splicing of I-6 is independent of functional FRQ. _frq_9 was grown in LL at the indicated temperatures. RNA was prepared, and splicing of I-6 was determined.
Figure 3.
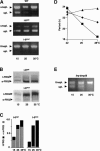
Splice-site mutations affecting thermosensitive splicing of I-6. The splice sites of I-6 were altered by site-directed mutagenesis toward consensus (I-6opt) and to nonsplice sites (I-6mut) as indicated in Supplementary Table 1. (A) Reverse transcription and qualitative PCR analysis showing that I-6opt is efficiently spliced at all temperatures while I-6mut is not spliced. (B) l-FRQ is the major protein product synthesized from the I-6mut allele and s-FRQ from I-6opt. (C) Quantification of Western blots shown in B. (Black bars) l-FRQ; (gray bars) s-FRQ. (D) The free-running period of I-6opt (triangles), I-6mut (squares), and wild-type (diamonds) control strains was determined on race tubes at the indicated temperatures. (E) I-8 of the Drosophila per gene (dmpi8) was inserted into the unique AflII site of the frq gene. The _frq_-dmpi8 allele was expressed in a _frq_10 background. Reverse transcription and qualitative PCR analysis showing that dmpi8 is spliced in a thermosensitive fashion in Neurospora.
Figure 4.
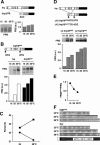
uORFs restrict translation of FRQ. (A) In _frq_ΔUTR residues, 50-1477 of the 5′-UTR are deleted and an AscI site is introduced. Western blot and densitometric quantification showing temperature-dependent expression of FRQ-encoded _frq_ΔUTR. (B) Construction _frq_1/6mut: Start codons of uORF1 and uORF6 were altered by site-directed mutagenesis to GTG. Temperature-dependent expression of FRQ encoded by frq and _frq_1/6mut is shown. (C) The free-running period of _frq_1/6mut (squares) and wild-type (circles) control (frq) was determined on race tubes at 25°C and 28°C. (D) Construction of ΔI-2 _frq_1/6AUG and ΔI-2 _frq_1/6mut: cDNA derived from I-2 spliced frq RNA was used as template for PCR reactions. Fragments including the translation initiation site of uORF1 and uORF6 were amplified by PCR and inserted into the AscI site of _frq_ΔUTR. Start codons of uORF1 and uORF6 were either left unchanged (ΔI-2 frq_1/6AUG) or changed to TTG and ACG (ΔI-2 frq_1/6mut). In the constructs shown, the C terminus of uORF6 (... HK_LLERE) was changed to... HK_GAPSSIAGF. (E) _frq_6cons: The translation initiation site of uORF6 was altered toward consensus (CAACATGG), and expression of FRQ was measured at the indicated temperatures. The ratios of FRQ expressed by _frq_6cons vs. FRQ expressed by a corresponding wild-type frq allele are shown. (F) Conidiation of the strain _frq_6cons. Race tubes were inoculated in LL and incubated at 25°C in DD. After 24 h, they were transferred to the indicated temperatures.
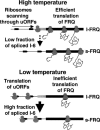
Figure 5.
Model for temperature sensing by the circadian clock via nonconsensus splicing of frq RNA and nonconsensus translation initiation at uORFs. For details, see Discussion.
Similar articles
- The molecular workings of the Neurospora biological clock.
Froehlich AC, Pregueiro A, Lee K, Denault D, Colot H, Nowrousian M, Loros JJ, Dunlap JC. Froehlich AC, et al. Novartis Found Symp. 2003;253:184-98; discussion 102-9, 198-202, 281-4. Novartis Found Symp. 2003. PMID: 14712922 Review. - Negative feedback defining a circadian clock: autoregulation of the clock gene frequency.
Aronson BD, Johnson KA, Loros JJ, Dunlap JC. Aronson BD, et al. Science. 1994 Mar 18;263(5153):1578-84. doi: 10.1126/science.8128244. Science. 1994. PMID: 8128244 - Long and short isoforms of Neurospora clock protein FRQ support temperature-compensated circadian rhythms.
Diernfellner A, Colot HV, Dintsis O, Loros JJ, Dunlap JC, Brunner M. Diernfellner A, et al. FEBS Lett. 2007 Dec 22;581(30):5759-64. doi: 10.1016/j.febslet.2007.11.043. Epub 2007 Nov 26. FEBS Lett. 2007. PMID: 18037381 Free PMC article. - Lithium leads to an increased FRQ protein stability and to a partial loss of temperature compensation in the Neurospora circadian clock.
Jolma IW, Falkeid G, Bamerni M, Ruoff P. Jolma IW, et al. J Biol Rhythms. 2006 Oct;21(5):327-34. doi: 10.1177/0748730406292453. J Biol Rhythms. 2006. PMID: 16998153 - The neurospora circadian system.
Dunlap JC, Loros JJ. Dunlap JC, et al. J Biol Rhythms. 2004 Oct;19(5):414-24. doi: 10.1177/0748730404269116. J Biol Rhythms. 2004. PMID: 15534321 Review.
Cited by
- Identification and expression of functionally conserved circadian clock genes in lichen-forming fungi.
Valim HF, Dal Grande F, Otte J, Singh G, Merges D, Schmitt I. Valim HF, et al. Sci Rep. 2022 Sep 23;12(1):15884. doi: 10.1038/s41598-022-19646-y. Sci Rep. 2022. PMID: 36151124 Free PMC article. - Ribonucleoprotein complexes that control circadian clocks.
Wang D, Liang X, Chen X, Guo J. Wang D, et al. Int J Mol Sci. 2013 Apr 25;14(5):9018-36. doi: 10.3390/ijms14059018. Int J Mol Sci. 2013. PMID: 23698761 Free PMC article. Review. - Temperature-modulated alternative splicing and promoter use in the Circadian clock gene frequency.
Colot HV, Loros JJ, Dunlap JC. Colot HV, et al. Mol Biol Cell. 2005 Dec;16(12):5563-71. doi: 10.1091/mbc.e05-08-0756. Epub 2005 Sep 29. Mol Biol Cell. 2005. PMID: 16195340 Free PMC article. - Differential regulation of phosphorylation, structure, and stability of circadian clock protein FRQ isoforms.
Chen X, Liu X, Gan X, Li S, Ma H, Zhang L, Wang P, Li Y, Huang T, Yang X, Fang L, Liang Y, Wu J, Chen T, Zhou Z, Liu X, Guo J. Chen X, et al. J Biol Chem. 2023 Apr;299(4):104597. doi: 10.1016/j.jbc.2023.104597. Epub 2023 Mar 9. J Biol Chem. 2023. PMID: 36898580 Free PMC article. - Splicing the Clock to Maintain and Entrain Circadian Rhythms.
Shakhmantsir I, Sehgal A. Shakhmantsir I, et al. J Biol Rhythms. 2019 Dec;34(6):584-595. doi: 10.1177/0748730419868136. Epub 2019 Aug 7. J Biol Rhythms. 2019. PMID: 31389290 Free PMC article. Review.
References
- Allada R., Emery, P., Takahashi, J.S., and Rosbash, M. 2001. Stopping time: The genetics of fly and mouse circadian clocks. Annu. Rev. Neurosci. 24: 1091-1119. - PubMed
- Bruchez J.J.P., Eberle, J., and Russo, V.E.A. 1993. Regulatory sequences involved in the translation of Neurospora crassa mRNA: Kozak sequences and stop codons. Fungal Genet. Newslett. 40: 85-88.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources