Conditional telomerase induction causes proliferation of hair follicle stem cells - PubMed (original) (raw)
Conditional telomerase induction causes proliferation of hair follicle stem cells
Kavita Y Sarin et al. Nature. 2005.
Abstract
TERT, the protein component of telomerase, serves to maintain telomere function through the de novo addition of telomere repeats to chromosome ends, and is reactivated in 90% of human cancers. In normal tissues, TERT is expressed in stem cells and in progenitor cells, but its role in these compartments is not fully understood. Here we show that conditional transgenic induction of TERT in mouse skin epithelium causes a rapid transition from telogen (the resting phase of the hair follicle cycle) to anagen (the active phase), thereby facilitating robust hair growth. TERT overexpression promotes this developmental transition by causing proliferation of quiescent, multipotent stem cells in the hair follicle bulge region. This new function for TERT does not require the telomerase RNA component, which encodes the template for telomere addition, and therefore operates through a mechanism independent of its activity in synthesizing telomere repeats. These data indicate that, in addition to its established role in extending telomeres, TERT can promote proliferation of resting stem cells through a non-canonical pathway.
Figures
Figure 1
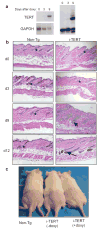
Conditional activation of TERT promotes the anagen phase of the hair follicle cycle. a, Diagram of hair follicle cycling b, TRAP on Non-Tg skin samples at days 4, 10 (anagen-A), 16 (catagen-C), 19, 21 (telogen-T), 28, 34 (A), and 52 (T). c–d, Northern blot analysis and TRAP on skin extracts from i-TERT and WT mice at day 50. e, Non-Tg (+doxy) and i-TERT (+doxy) mice at day 70. f, H&E skin sections (*=telogen hair follicle, arrow= anagen hair follicle), 20x. g, RNA in situ hybridization for TERT mRNA and immunofluorescence for K14 in i-TERT (+doxy) skin (*=autofluorescence). h, RNA in situ hybridization for TERT mRNA (blue) in i-TERT(+doxy) skin section (right) and WT anagen skin (left) (*=dermal papilla).
Figure 2
TERT induction triggers a rapid transition from telogen to anagen. a, Northern blot (left) and TRAP assay (right) show increased TERT expression and telomerase activity by nine days of doxycycline treatment. b, Follicles in i-TERT mice entered anagen (arrow) by day 9, whereas Non-Tg controls remained in telogen (*), H&E, 20x. c, Hair growth was observed only in i-TERT mice (+doxy) (right), but not in i-TERT mice (−doxy) (center) or Non-Tg littermates (left).
Figure 3
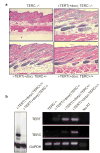
TERT activates stem cells, depleting BrdU label from LRCs. a, Immunofluorescence for BrdU (red) and CD34 (green) shows maintenance of LRCs in Non-Tg group, but dramatic loss of label in i-TERT mice after doxy treatment (pre-doxy = day 55, post-doxy= day 90). b, Quantification of LRC data from (a), showing number of BrdU+ cells/CD34+ cells. i-TERT (black bars, n=4 mice), Non-Tg (gray bars, n=3 mice), - indicates pre-doxy, + indicates post-doxy. c, LRC analysis from whole mounts of epidermis from tail of mice labeled with BrdU at day 10, switched to doxy at day 40 and analyzed at day 65. (BrdU=red, K14=green). d, Immunofluorescence using Ki-67 (red) to mark proliferating cells and K14 (green) to identify basal layer of skin. e, Quantitation of proliferation index in (d) as Ki-67+ cells/100μm length of basal layer. n=2 mice for each comparison. f, GFP epifluorescence costained with CD34 (inset, confocal microscopy) in skin section from an actin-GFP mouse. g, RNA in situ analysis for TERT mRNA in i-TERT(+doxy) mouse skin. (inset) TERT mRNA expression (cytoplasmic) overlaps in bulge with LRCs, marked by BrdU (nuclear). h, H&E sections from K5tTA+; tetop-TERT+ (−doxy) (bottom) and Non-Tg (top) mice, 20X. Error bars indicate standard deviation. p values based on t-test. *=autofluorescence of hair.
Figure 4
TERT's activity in facilitating a transition from telogen to anagen is independent of its function in telomere synthesis. a, TERT induced anagen in mice with TERC+/+, TERC+/−, and TERC−/− backgrounds, H&E 20x. b, Skin samples from i-TERT; TERC−/− mice lacked telomerase activity by TRAP (left) and lacked TERC expression by RT-PCR (right).
Comment in
- Cell biology: Shaggy mouse tales.
Blackburn EH. Blackburn EH. Nature. 2005 Aug 18;436(7053):922-3. doi: 10.1038/436922a. Nature. 2005. PMID: 16107824 No abstract available.
Similar articles
- Cell biology: Shaggy mouse tales.
Blackburn EH. Blackburn EH. Nature. 2005 Aug 18;436(7053):922-3. doi: 10.1038/436922a. Nature. 2005. PMID: 16107824 No abstract available. - TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program.
Choi J, Southworth LK, Sarin KY, Venteicher AS, Ma W, Chang W, Cheung P, Jun S, Artandi MK, Shah N, Kim SK, Artandi SE. Choi J, et al. PLoS Genet. 2008 Jan;4(1):e10. doi: 10.1371/journal.pgen.0040010. Epub 2007 Dec 13. PLoS Genet. 2008. PMID: 18208333 Free PMC article. - Effects of telomerase and telomere length on epidermal stem cell behavior.
Flores I, Cayuela ML, Blasco MA. Flores I, et al. Science. 2005 Aug 19;309(5738):1253-6. doi: 10.1126/science.1115025. Epub 2005 Jul 21. Science. 2005. PMID: 16037417 - Dissecting the non-canonical functions of telomerase.
Parkinson EK, Fitchett C, Cereser B. Parkinson EK, et al. Cytogenet Genome Res. 2008;122(3-4):273-80. doi: 10.1159/000167813. Epub 2009 Jan 30. Cytogenet Genome Res. 2008. PMID: 19188696 Review. - Telomere Dynamics and Telomerase in the Biology of Hair Follicles and their Stem Cells as a Model for Aging Research.
Stone RC, Aviv A, Paus R. Stone RC, et al. J Invest Dermatol. 2021 Apr;141(4S):1031-1040. doi: 10.1016/j.jid.2020.12.006. Epub 2021 Jan 26. J Invest Dermatol. 2021. PMID: 33509633 Review.
Cited by
- Telomerase and telomere length in pulmonary fibrosis.
Liu T, Ullenbruch M, Young Choi Y, Yu H, Ding L, Xaubet A, Pereda J, Feghali-Bostwick CA, Bitterman PB, Henke CA, Pardo A, Selman M, Phan SH. Liu T, et al. Am J Respir Cell Mol Biol. 2013 Aug;49(2):260-8. doi: 10.1165/rcmb.2012-0514OC. Am J Respir Cell Mol Biol. 2013. PMID: 23526226 Free PMC article. Clinical Trial. - VEGF signaling has distinct spatiotemporal roles during heart valve development.
Stankunas K, Ma GK, Kuhnert FJ, Kuo CJ, Chang CP. Stankunas K, et al. Dev Biol. 2010 Nov 15;347(2):325-36. doi: 10.1016/j.ydbio.2010.08.030. Epub 2010 Sep 15. Dev Biol. 2010. PMID: 20816797 Free PMC article. - Neuronal nitric oxide synthase and affective disorders.
Zhou QG, Zhu XH, Nemes AD, Zhu DY. Zhou QG, et al. IBRO Rep. 2018 Nov 17;5:116-132. doi: 10.1016/j.ibror.2018.11.004. eCollection 2018 Dec. IBRO Rep. 2018. PMID: 30591953 Free PMC article. Review. - Cell biology: The not-so-odd couple.
Millar SE. Millar SE. Nature. 2009 Jul 2;460(7251):44-5. doi: 10.1038/460044a. Nature. 2009. PMID: 19571874 No abstract available. - Healthy aging and disease: role for telomere biology?
Zhu H, Belcher M, van der Harst P. Zhu H, et al. Clin Sci (Lond). 2011 May;120(10):427-40. doi: 10.1042/CS20100385. Clin Sci (Lond). 2011. PMID: 21271986 Free PMC article. Review.
References
- Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–73. - PubMed
- Smogorzewska A, De Lange T. Regulation of telomerase by telomeric proteins. Annu Rev Biochem. 2004;73:177–208. - PubMed
- Morrison SJ, Prowse KR, Ho P, Weissman IL. Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity. 1996;5:207–216. - PubMed
- Yui J, Chiu CP, Lansdorp PM. Telomerase activity in candidate stem cells from fetal liver and adult bone marrow. Blood. 1998;91:3255–62. - PubMed
- Forsyth NR, Wright WE, Shay JW. Telomerase and differentiation in multicellular organisms: turn it off, turn it on, and turn it off again. Differentiation. 2002;69:188–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases