Focal adhesions as mechanosensors: a physical mechanism - PubMed (original) (raw)
Focal adhesions as mechanosensors: a physical mechanism
Tom Shemesh et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Focal adhesions (FA) are large, multiprotein complexes that provide a mechanical link between the cytoskeletal contractile machinery and the extracellular matrix. FA exhibit mechanosensitive properties; they self-assemble and elongate upon application of pulling forces and dissociate when these forces are decreased. We propose a thermodynamic model for the mechanosensitivity of FA, according to which a molecular aggregate, subjected to pulling forces, tends to grow in the direction of force application by incorporating additional molecules. We demonstrate that this principle is consistent with the phenomenology of FA dynamics by considering a one-dimensional protein aggregate subjected to pulling forces and anchored to the substrate. Depending on the force level, force distribution along the aggregate, and the character of its anchoring to the substrate, the aggregate is predicted to exhibit distinct modes of assembly that are largely consistent with the experimentally observed FA behavior. We define here specific conditions that can lead to the different regimes of FA assembly, including growth, steady state, and disassembly.
Figures
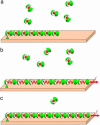
Fig. 1.
Schematic representation of self-assembly upon pulling force. (a) The protein aggregate and the free proteins in the surrounding medium. (b) Application of the pulling force results in the aggregate stretching and the related accumulation of the elastic stresses within the aggregate. (c) Insertion of new proteins into the aggregate results in stress relaxation.
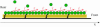
Fig. 2.
A 1D aggregate subject to pulling forces f and anchored to substrate, showing an illustration of the model. The points of force application and the points of anchoring are distributed along the aggregate surface, each with its own density.
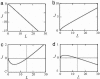
Fig. 3.
Four regimes of self-assembly determined by the parameter χ = (_f_·_l_0)/(Δμ0). The curves represent the total protein flux, J(arbitrary units), to the aggregate as a function of the aggregate length, L(arbitrary units). (a) Atχ < 1, and χ < χ*, the flux is negative, meaning that the aggregate undergoes disintegration. (_b_) At χ > 1, and χ > χ*, the flux is positive, meaning that the aggregate undergoes unlimited growth. (c) At χ < 1 but χ > χ*, the flux is negative until the aggregate reaches a certain length where the flux changes sign and starts increasing with the aggregate length. This regime corresponds to unlimited growth after overcome of a critical length. (d) At χ > 1 and χ <χ*, the flux remains positive until the aggregate reaches a critical length. For length larger than the critical values, the flux is negative. This state is the steady-state regime, where the critical length corresponds to the stable steady-state length of the aggregate.
Fig. 4.
Phase diagram of the system representing the different regimes of the aggregate assembly–disassembly corresponding to different ranges of the system parameters, which are the density of the points of force application along the aggregate length ϕ_f_, the density of the points of the aggregate anchoring to the substrate ϕ_A_, and the dimensionless parameter χ = (_f_·_l_0)/(Δμ0), characterizing the ratio between the molecular energy provided by an elementary pulling force, _f_·_l_0, and the difference of the protein standard chemical potentials in the aggregated and free states, Δμ0.
Similar articles
- Assembly and mechanosensory function of focal adhesions: experiments and models.
Bershadsky AD, Ballestrem C, Carramusa L, Zilberman Y, Gilquin B, Khochbin S, Alexandrova AY, Verkhovsky AB, Shemesh T, Kozlov MM. Bershadsky AD, et al. Eur J Cell Biol. 2006 Apr;85(3-4):165-73. doi: 10.1016/j.ejcb.2005.11.001. Epub 2005 Dec 19. Eur J Cell Biol. 2006. PMID: 16360240 Review. - Focal adhesions as mechanosensors: the two-spring model.
Schwarz US, Erdmann T, Bischofs IB. Schwarz US, et al. Biosystems. 2006 Feb-Mar;83(2-3):225-32. doi: 10.1016/j.biosystems.2005.05.019. Epub 2005 Oct 19. Biosystems. 2006. PMID: 16236431 - Cell mechanosensitivity controls the anisotropy of focal adhesions.
Nicolas A, Geiger B, Safran SA. Nicolas A, et al. Proc Natl Acad Sci U S A. 2004 Aug 24;101(34):12520-5. doi: 10.1073/pnas.0403539101. Epub 2004 Aug 16. Proc Natl Acad Sci U S A. 2004. PMID: 15314229 Free PMC article. - Force meets chemistry: analysis of mechanochemical conversion in focal adhesions using fluorescence recovery after photobleaching.
Lele TP, Thodeti CK, Ingber DE. Lele TP, et al. J Cell Biochem. 2006 Apr 15;97(6):1175-83. doi: 10.1002/jcb.20761. J Cell Biochem. 2006. PMID: 16408278 Review. - Force-induced adsorption and anisotropic growth of focal adhesions.
Besser A, Safran SA. Besser A, et al. Biophys J. 2006 May 15;90(10):3469-84. doi: 10.1529/biophysj.105.074377. Epub 2006 Mar 2. Biophys J. 2006. PMID: 16513789 Free PMC article.
Cited by
- Review of cellular mechanotransduction on micropost substrates.
Geng Y, Wang Z. Geng Y, et al. Med Biol Eng Comput. 2016 Mar;54(2-3):249-71. doi: 10.1007/s11517-015-1343-2. Epub 2015 Aug 6. Med Biol Eng Comput. 2016. PMID: 26245253 Review. - Dynamic regulation of the structure and functions of integrin adhesions.
Wolfenson H, Lavelin I, Geiger B. Wolfenson H, et al. Dev Cell. 2013 Mar 11;24(5):447-58. doi: 10.1016/j.devcel.2013.02.012. Dev Cell. 2013. PMID: 23484852 Free PMC article. Review. - Cytoskeletal bundle mechanics.
Bathe M, Heussinger C, Claessens MM, Bausch AR, Frey E. Bathe M, et al. Biophys J. 2008 Apr 15;94(8):2955-64. doi: 10.1529/biophysj.107.119743. Epub 2007 Nov 30. Biophys J. 2008. PMID: 18055529 Free PMC article. - Mapping the dynamics of shear stress-induced structural changes in endothelial cells.
Mott RE, Helmke BP. Mott RE, et al. Am J Physiol Cell Physiol. 2007 Nov;293(5):C1616-26. doi: 10.1152/ajpcell.00457.2006. Epub 2007 Sep 13. Am J Physiol Cell Physiol. 2007. PMID: 17855768 Free PMC article. - Stability of adhesion clusters and cell reorientation under lateral cyclic tension.
Kong D, Ji B, Dai L. Kong D, et al. Biophys J. 2008 Oct;95(8):4034-44. doi: 10.1529/biophysj.108.131342. Epub 2008 Jul 11. Biophys J. 2008. PMID: 18621806 Free PMC article.
References
- Bershadsky, A. D., Balaban, N. Q. & Geiger, B. (2003) Annu. Rev. Cell Dev. Biol. 19, 677–695. - PubMed
- Geiger, B. & Bershadsky, A. (2001) Curr. Opin. Cell Biol. 13, 584–592. - PubMed
- Geiger, B. & Bershadsky, A. (2002) Cell 110, 139–142. - PubMed
- Zamir, E. & Geiger, B. (2001) J. Cell Sci. 114, 3583–3590. - PubMed
- Zamir, E. & Geiger, B. (2001) J. Cell Sci. 114, 3577–3579. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources