p75 neurotrophin receptor reduces ligand-induced Trk receptor ubiquitination and delays Trk receptor internalization and degradation - PubMed (original) (raw)
p75 neurotrophin receptor reduces ligand-induced Trk receptor ubiquitination and delays Trk receptor internalization and degradation
Joe P S Makkerh et al. EMBO Rep. 2005 Oct.
Abstract
Target-derived neurotrophins regulate neuronal survival and growth by interacting with cell-surface tyrosine kinase receptors. The p75 neurotrophin receptor (p75 NTR) is coexpressed with Trk receptors in long-range projection neurons, in which it facilitates neurotrophin binding to Trk and enhances Trk activity. Here, we show that TrkA and TrkB receptors undergo robust ligand-dependent ubiquitination that is dependent on activation of the endogenous Trk activity of the receptors. Coexpression of p75 NTR attenuated ubiquitination of TrkA and TrkB and delayed nerve growth factor-induced TrkA receptor internalization and receptor degradation. These results indicate that p75 NTR may prolong cell-surface Trk-dependent signalling events by negatively regulating receptor ubiquitination.
Figures
Figure 1
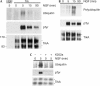
Treatment of mouse cortical neurons with brain-derived neurotrophic factor (BDNF) induces ubiquitination of TrkB. (A) Mouse cortical neurons were treated with 50 ng/ml BDNF for 5–90 min and TrkB was immunoprecipitated and immunoblotted for total ubiquitin and phosphotyrosine (pTyr) content, as indicated. (B) Cortical neurons were treated with BDNF or nerve growth factor (NGF) in the absence or presence of 200 ng/ml K252A for 5 or 15 min, as indicated, and TrkB was immunoprecipitated and analysed as above. (C) Cortical neurons were treated with BDNF (50 ng/ml) for 5–90 min and TrkB was immunoprecipitated. Immunoprecipitates were analysed by immunoblotting for phosphotyrosine, total ubiquitin and polyubiquitin content and for TrkB levels using 4G10, P4D1, FK1 and anti-TrkB (RTB) antibodies, respectively. Experiments in (A–C) were each performed three times, all with identical results.
Figure 2
Nerve growth factor (NGF) induces modest TrkA ubiquitination in PC12 cells. (A) PC12 cells were treated with 50 ng/ml NGF for 5–90 min, as indicated, and TrkA was immunoprecipitated and analysed by immunoblotting for total ubiquitin and phosphotyrosine (pTyr) content and for TrkA levels, as indicated. NSR, nonspecific rabbit sera. (B) PC12 cells were treated with 50 ng/ml NGF for 5–90 min and TrkA was immunoprecipitated. Immunoprecipitates were analysed by immunoblotting for polyubiquitin content (using the FK1 antibody), phosphotyrosine content and TrkA levels, as indicated. (C) PC12 cells were treated with NGF in the absence or presence of 200 ng/ml K252A for 5 min and TrkA was immunoprecipitated and analysed by immunoblotting for phosphotyrosine, total ubiquitin and TrkA, as indicated. Experiments in (A,B) were each performed three times, and the experiment in (C) was performed twice, all with identical results.
Figure 3
p75 neurotrophin receptor (p75NTR) inhibits ligand-induced Trk receptor ubiquitination. (A) Human embryonic kidney 293 cells were transfected with expression plasmids encoding TrkA and Myc-tagged ubiquitin (Myc–Ubq) in the presence and absence of an expression plasmid encoding p75NTR. At 2 days after the transfection, cells were treated with nerve growth factor (NGF; 50 ng/ml) for 5 or 90 min and then lysed in harsh denaturing conditions. Ubiquitinated proteins were immunoprecipitated (IP) using antibodies directed against the Myc epitope and analysed on immunoblots using antibodies directed against TrkA and p75NTR. Lysate levels of TrkA and p75NTR are shown in the lower panels. (B) Transfections performed as in (A) were followed by immunoprecipitations using an anti-TrkA antibody (203) or using a rabbit antibody control (Ig). Immunoblots for total ubiquitin and polyubiquitin content were performed using P4D1 and FK1 antibodies, respectively. (C) Experiments were performed as in (A), except that the expression plasmid encoding TrkA was replaced with a TrkB expression plasmid and cells were treated with 50 ng/ml brain-derived neurotrophic factor (BDNF) for 5 min. Lysate levels of TrkB and p75NTR are shown in the lower panels. (D) Experiments were performed as in (A), except that the expression plasmid encoding p75NTR was replaced with an expression plasmid encoding a mutant form of p75NTR (p75NTR-Tr) lacking cysteine-rich domains 2–4 and cells were treated with 50 ng/ml NGF for 5 min only. pTyr, phosphotyrosine. (E) The PC12p75kd line and the control PC12 line (stably transfected with green fluorescent protein (GFP) and maintained in G418) were treated with NGF (50 ng/ml) for 5–90 min and TrkA was immunoprecipitated. Immunoprecipitates were analysed by immunoblotting for TrkA levels and for total ubiquitin and phosphotyrosine content, as indicated. p75NTR levels in the two lines are shown in the lower panel. GFP, green fluorescent protein; RNAi, RNA interference. Experiments in (A–F) were each performed three times, all with identical results.
Figure 4
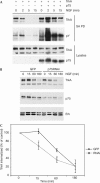
p75NTR inhibits ligand-induced tyrosine kinase (Trk) receptor ubiquitination. (A) Human embryonic kidney 293 cells were transfected with expression plasmids encoding TrkA and p75 neurotrophin receptor (p75NTR), and after 2 days were exposed to nerve growth factor (NGF; 50 ng/ml) at 37°C for 2–15 min, as indicated. Cellsurface proteins were then biotinylated at 4°C, biotinylated proteins were precipitated from lysed cells using streptavidin beads and levels of TrkA and the TrkA phosphotyrosine (pTyr) content were determined by immunoblotting. Lysate levels of TrkA and p75NTR are shown in the lower panels. SA PD, streptavidin pullot. (B) The PC12p75kd line and the control PC12 line were biotinylated at 4°C, exposed to 50 ng/ml NGF and then incubated at 37°C for the durations indicated. Biotinylated proteins were recovered from lysed cells using streptavidin-coated beads and levels of TrkA in the precipitates were determined by immunoblotting using RTA. Lysate levels of p75NTR and Erk are shown in the lower panels. GFP, green fluorescent protein; RNAi, RNA interference. (C) Quantification of data shown in (B) from three separate experiments determined by densitometry, pooled and analysed for significance by one-way analysis of variance. *P<0.05. The experiment in (A) was performed three times, all with identical results.
Similar articles
- p75 Co-receptors regulate ligand-dependent and ligand-independent Trk receptor activation, in part by altering Trk docking subdomains.
Zaccaro MC, Ivanisevic L, Perez P, Meakin SO, Saragovi HU. Zaccaro MC, et al. J Biol Chem. 2001 Aug 17;276(33):31023-9. doi: 10.1074/jbc.M104630200. Epub 2001 Jun 25. J Biol Chem. 2001. PMID: 11425862 - Trk receptors need neutral sphingomyelinase activity to promote cell viability.
Candalija A, Cubí R, Ortega A, Aguilera J, Gil C. Candalija A, et al. FEBS Lett. 2014 Jan 3;588(1):167-74. doi: 10.1016/j.febslet.2013.11.032. Epub 2013 Dec 5. FEBS Lett. 2014. PMID: 24316227 - The effect of P75 on Trk receptors in neuroblastomas.
Ho R, Minturn JE, Simpson AM, Iyer R, Light JE, Evans AE, Brodeur GM. Ho R, et al. Cancer Lett. 2011 Jun 1;305(1):76-85. doi: 10.1016/j.canlet.2011.02.029. Epub 2011 Mar 17. Cancer Lett. 2011. PMID: 21419569 Free PMC article. - A, B, C's of Trk Receptors and Their Ligands in Ocular Repair.
Gupta A, Galletti JG, Yu Z, Burgess K, de Paiva CS. Gupta A, et al. Int J Mol Sci. 2022 Nov 15;23(22):14069. doi: 10.3390/ijms232214069. Int J Mol Sci. 2022. PMID: 36430547 Free PMC article. Review. - Circadian control of p75 neurotrophin receptor leads to alternate activation of Nrf2 and c-Rel to reset energy metabolism in astrocytes via brain-derived neurotrophic factor.
Ishii T, Warabi E, Mann GE. Ishii T, et al. Free Radic Biol Med. 2018 May 1;119:34-44. doi: 10.1016/j.freeradbiomed.2018.01.026. Epub 2018 Jan 31. Free Radic Biol Med. 2018. PMID: 29374533 Review.
Cited by
- NGF-induced TrkA/CD44 association is involved in tumor aggressiveness and resistance to lestaurtinib.
Aubert L, Guilbert M, Corbet C, Génot E, Adriaenssens E, Chassat T, Bertucci F, Daubon T, Magné N, Le Bourhis X, Toillon RA. Aubert L, et al. Oncotarget. 2015;6(12):9807-19. doi: 10.18632/oncotarget.3227. Oncotarget. 2015. PMID: 25840418 Free PMC article. - Non-canonical Ret signaling augments p75-mediated cell death in developing sympathetic neurons.
Donnelly CR, Gabreski NA, Suh EB, Chowdhury M, Pierchala BA. Donnelly CR, et al. J Cell Biol. 2018 Sep 3;217(9):3237-3253. doi: 10.1083/jcb.201703120. Epub 2018 Jul 17. J Cell Biol. 2018. PMID: 30018091 Free PMC article. - Enhanced TrkA signaling impairs basal forebrain-dependent behavior.
Calvo-Enrique L, Lisa S, Vicente-García C, Deogracias R, Arévalo JC. Calvo-Enrique L, et al. Front Mol Neurosci. 2023 Sep 22;16:1266983. doi: 10.3389/fnmol.2023.1266983. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37808473 Free PMC article. - Nerve growth factor modulates the tumor cells migration in ovarian cancer through the WNT/β-catenin pathway.
Li B, Cai S, Zhao Y, He Q, Yu X, Cheng L, Zhang Y, Hu X, Ke M, Chen S, Zou M. Li B, et al. Oncotarget. 2016 Dec 6;7(49):81026-81048. doi: 10.18632/oncotarget.13186. Oncotarget. 2016. PMID: 27835587 Free PMC article. - Mouse Nerve Growth Factor Facilitates the Growth of Interspinal Schwannoma Cells by Activating NGF Receptors.
Liu SY, Liu SZ, Li Y, Chen S. Liu SY, et al. J Korean Neurosurg Soc. 2019 Nov;62(6):626-634. doi: 10.3340/jkns.2019.0081. Epub 2019 Sep 18. J Korean Neurosurg Soc. 2019. PMID: 31527385 Free PMC article.
References
- Berg MM, Sternberg DW, Parada LF, Chao MV (1992) K252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. J Biol Chem 267: 13–16 - PubMed
- Delcroix JD, Valletta JS, Wu C, Hunt SJ, Kowal AS, Mobley WC (2003) NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron 39: 69–84 - PubMed
- Di Fiore PP, De Camilli P (2001) Endocytosis and signaling. An inseparable partnership. Cell 106: 1–4 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials