Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells - PubMed (original) (raw)
Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells
Jan Lammerding et al. J Cell Biol. 2005.
Abstract
Emery-Dreifuss muscular dystrophy can be caused by mutations in the nuclear envelope proteins lamin A/C and emerin. We recently demonstrated that A-type lamin-deficient cells have impaired nuclear mechanics and altered mechanotransduction, suggesting two potential disease mechanisms (Lammerding, J., P.C. Schulze, T. Takahashi, S. Kozlov, T. Sullivan, R.D. Kamm, C.L. Stewart, and R.T. Lee. 2004. J. Clin. Invest. 113:370-378). Here, we examined the function of emerin on nuclear mechanics and strain-induced signaling. Emerin-deficient mouse embryo fibroblasts have abnormal nuclear shape, but in contrast to A-type lamin-deficient cells, exhibit nuclear deformations comparable to wild-type cells in cellular strain experiments, and the integrity of emerin-deficient nuclear envelopes appeared normal in a nuclear microinjection assay. Interestingly, expression of mechanosensitive genes in response to mechanical strain was impaired in emerin-deficient cells, and prolonged mechanical stimulation increased apoptosis in emerin-deficient cells. Thus, emerin-deficient mouse embryo fibroblasts have apparently normal nuclear mechanics but impaired expression of mechanosensitive genes in response to strain, suggesting that emerin mutations may act through altered transcriptional regulation and not by increasing nuclear fragility.
Figures
Figure 1.
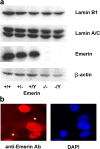
Characterization of emerin null fibroblasts. (a) Western analysis of homozygous (−/− and −/Y) and heterozygous (+/−) emerin null and wild-type (+/+ and +/Y) adult muscle fibroblasts, showing a complete lack of emerin in the homozygous cells. Protein expression of lamin A/C and lamin B1 appeared normal in the emerin null homozygous and heterozygous cells. (b) Female heterozygous (i.e., Emd +/−) muscle fibroblasts stained with anti-emerin antibody (left) and DAPI (right). Two cells (*) clearly lack emerin staining (as visualized by comparing with the DAPI staining), reflecting the differential X-chromosome inactivation in the heterozygous cell population.
Figure 2.
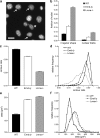
Emerin and A-type lamin-deficient fibroblasts have abnormal nuclear shape. (a) Fluorescently labeled nuclei of emerin-deficient mouse embryo fibroblasts. Asterisk denotes nucleus with chromatin protruding from the nucleus (nuclear bleb). Arrows indicate nuclei that have mild deviations from the typical round shape. Bar, 20 μm. (b) The fraction of abnormally shaped nuclei and nuclear blebs was significantly increased in emerin and A-type lamin-deficient fibroblasts, with emerin-deficient cells displaying a milder phenotype (cell fractions with abnormal nuclear shape were 0.077 ± 0.006 for wild-type, 0.163 ± 0.009 for emerin-deficient, and 0.378 ± 0.011 for A-type lamin-deficient cells, P < 0.0001 for emerin-deficient and A-type lamin-deficient cells compared with wild-type cells; cell fractions with nuclear blebs were 0.012 ± 0.002, 0.042 ± 0.005, and 0.093 ± 0.006 for wild-type, emerin-deficient, and A-type lamin-deficient cells, respectively, P < 0.0001 for emerin-deficient and A-type lamin-deficient cells compared with wild-type cells; n ∼1,800 for each cell type). (c) Emerin-deficient cells have a significantly decreased contour ratio compared with wild-type cells, but display a milder phenotype compared with A-type lamin-deficient cells (contour ratio = 0.89 ± 0.004 for wild-type, 0.86 ± 0.006 for emerin-deficient, and 0.80 ± 0.007 for A-type lamin-deficient cells, P < 0.001 for emerin-deficient and A-type lamin-deficient cells compared with wild-type; n ∼1500 for each cell type in three independent experiments). (d) Relative frequency distribution of the contour ratio for wild-type, emerin-deficient, and A-type lamin-deficient cells (median values were 0.896, 0.869, and 0.817 for wild-type, emerin-deficient, and A-type lamin-deficient cells, respectively). The difference in the medians was statistically significant (P < 0.001 for emerin-deficient and A-type lamin-deficient vs. wild-type). (e) Emerin and A-type lamin-deficient cells have significantly increased nuclear cross-sectional areas compared with wild-type cells (Nuclear cross-sectional area = 178 ± 4.5 μm2, 252 ± 24.7 μm2, and 259 ± 25.6 μm2 for wild-type, emerin-deficient, and A-type lamin-deficient cells, respectively, P < 0.01 for emerin and A-type lamin-deficient compared with wild-type cells, n ∼1500 for each cell type in three independent experiments). (f) Frequency distribution of the cross-sectional area for wild-type, emerin-deficient, and A-type lamin-deficient cells.
Figure 3.
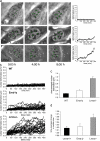
Emerin and A-type lamin-deficient cells have increased nuclear dynamics and decreased nuclear shape stability. (a) Time-lapse series of fibroblasts over an 8 h, 20 min time period. Images shown were acquired at 5 min, 4 h, and 8 h. Wild-type nuclei (top row) appear very stable over time and have only minor deformations, whereas A-type lamin-deficient nuclei (bottom row) show large nuclear deformations over time. Emerin-deficient nuclei (center row) display an intermediate phenotype, with some nuclei appearing very stable and other nuclei undergoing larger deformations. White crosses denote initial positions of nucleoli, green crosses positions according to the least-square fit assuming linear affine transformations (see Materials and methods), and black crosses the actual nucleoli centroid positions. Deviations between the black and green positions indicate nuclear deformations independent of translation, rotation, or uniform changes in nuclear size. Plots on the right show the average deviation between the actual nucleoli positions and the least-square fit. Time-lapse videos are available online at
http://www.jcb.org/cgi/content/full/jcb.200502148/DC1
. (b) Time courses of the nuclear deformations for wild-type (top), emerin-deficient (center), and A-type lamin-deficient (bottom) fibroblasts, 25 nuclei each. (c) Emerin-deficient cells have significantly increased time-averaged nuclear deformations compared with wild-type cells, but to a much lesser extent than A-type lamin-deficient cells (time-averaged nuclear deformation = 0.23 ± 0.015 μm, 0.42 ± 0.038 μm, and 1.11 ± 0.104 μm for wild-type, emerin-deficient, and A-type lamin-deficient cells, respectively; P < 0.0001 for emerin and A-type lamin-deficient vs. wild-type cells). (d) A-type lamin-deficient cells have significantly increased time-averaged nuclear size changes compared with wild-type and emerin-deficient cells (time-averaged normalized size change = 1.018 ± 0.006, 1.023 ± 0.010, and 1.058 ± 0.013 for wild-type, emerin-deficient, and A-type lamin-deficient cells, respectively; P < 0.01 for A-type lamin-deficient vs. wild-type cells).
Figure 4.
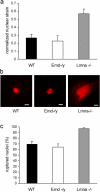
Emerin-deficient cells have apparently normal nuclear mechanics. (a) Emerin-deficient primary mouse embryo fibroblasts have normalized nuclear strain comparable to wild-type cells when subjected to biaxial strain. In contrast, A-type lamin-deficient cells have significantly increased normalized nuclear strain (normalized nuclear strain = 0.27 ± 0.044, 0.23 ± 0.066, and 0.57 ± 0.059 for wild-type, emerin-deficient, and A-type lamin-deficient cells respectively; P < 0.001 for A-type lamin-deficient vs. wild-type cells). (b) Wild-type (left) and emerin-deficient (center) nuclei remain intact when microinjected with fluorescently labeled dextran, whereas A-type lamin-deficient nuclei (right) have more fragile nuclei that allow dextran to leak into the cytoplasm. Bars, 20 μm. (c) Emerin-deficient and wild-type cells have comparable frequency of nuclear rupture for nuclear microinjection at 500 hPa, whereas A-type lamin-deficient cells have a significantly increased fraction of ruptured nuclei (percentage of ruptured nuclei = 69 ± 4.9%, 64 ± 5.5%, and 96 ± 1.9% for wild-type, emerin-deficient, and A-type lamin-deficient cells, respectively).
Figure 5.
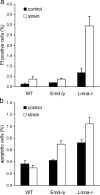
Emerin and A-type lamin-deficient cells are more sensitive to mechanical strain. (a) A-type lamin-deficient cells have a significantly increased number of propidium iodide–positive cells after 24 h of cyclic, 10% biaxial strain application. Emerin-deficient cells are not significantly different from wild-type cells. (Percentage of propidium iodide–positive cells at rest vs. after 24 h strain application = 0.13 ± 0.03% vs. 0.38 ± 0.12%, 0.2 ± 0.00% vs. 0.37 ± 0.04%, and 0.68 ± 0.18% vs. 2.94 ± 0.47% for wild-type, emerin-deficient, and A-type lamin-deficient cells respectively, P < 0.01 for wild-type vs. A-type lamin-deficient strained cells; P < 0.05 for wild-type vs. A-type lamin-deficient controls). (b) Emerin and A-type lamin-deficient cells have increased fractions of apoptotic cells after 24 h of cyclic, 10% biaxial strain application (percentage of apoptotic cells at rest vs. after 24 h of strain application = 0.37 ± 0.05% vs. 0.30 ± 0.04% for wild-type, 0.43 ± 0.02% vs. 0.70 ± 0.06% for emerin-deficient, and 0.72 ± 0.05% vs. 1.04 ± 0.11% for A-type lamin-deficient cells; P < 0.001 for strained emerin and A-type lamin-deficient vs. wild-type cells).
Figure 6.
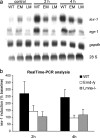
Emerin and A-type lamin-deficient cells have impaired mechanotransduction. (a) Expression of the mechanosensitive genes iex-1 and egr-1 in response to mechanical strain is drastically reduced in emerin and A-type lamin-deficient fibroblasts compared with wild-type cells. Expression of the nonmechanically inducible gene gapdh is not significantly altered. The elevated baseline expression of iex-1 and egr-1 seen in the emerin-deficient cells in this Northern blot are not representative, and real-time PCR analysis didn't reveal any significant differences in baseline expression between cell types. (b) Real-time PCR analysis confirms the impaired induction of iex-1 in response to strain in emerin and A-type lamin-deficient cells. Results are normalized to β-tubulin expression and presented as percent induction of baseline levels (induction at 2 h: 270 ± 87.1%, 143 ± 41.4%, and 104 ± 35.9% for wild-type, emerin-deficient, and A-type lamin-deficient cells, respectively, P < 0.05 for wild-type vs. A-type lamin-deficient; induction at 4 h: 244 ± 54.8%, 95 ± 20.3%, and 116 ± 39.8% for wild-type, emerin-deficient, and A-type lamin-deficient cells respectively, P < 0.05 for wild-type vs. emerin-deficient and wild-type vs. A-type lamin-deficient cells; differences in baseline expression were not statistically significant between any cell type).
Similar articles
- Effect of pathogenic mis-sense mutations in lamin A on its interaction with emerin in vivo.
Holt I, Ostlund C, Stewart CL, Man Nt, Worman HJ, Morris GE. Holt I, et al. J Cell Sci. 2003 Jul 15;116(Pt 14):3027-35. doi: 10.1242/jcs.00599. Epub 2003 Jun 3. J Cell Sci. 2003. PMID: 12783988 - Immunocytochemistry of nuclear domains and Emery-Dreifuss muscular dystrophy pathophysiology.
Maraldi NM, Lattanzi G, Sabatelli P, Ognibene A, Columbaro M, Capanni C, Rutigliano C, Mattioli E, Squarzoni S. Maraldi NM, et al. Eur J Histochem. 2003;47(1):3-16. doi: 10.4081/802. Eur J Histochem. 2003. PMID: 12685553 Review. - How does a g993t mutation in the emerin gene cause Emery-Dreifuss muscular dystrophy?
Holt I, Clements L, Manilal S, Morris GE. Holt I, et al. Biochem Biophys Res Commun. 2001 Oct 12;287(5):1129-33. doi: 10.1006/bbrc.2001.5708. Biochem Biophys Res Commun. 2001. PMID: 11587540 - The nuclear membrane and mechanotransduction: impaired nuclear mechanics and mechanotransduction in lamin A/C deficient cells.
Lammerding J, Lee RT. Lammerding J, et al. Novartis Found Symp. 2005;264:264-73; discussion 273-8. Novartis Found Symp. 2005. PMID: 15773759 Review. - Chromosome positioning is largely unaffected in lymphoblastoid cell lines containing emerin or A-type lamin mutations.
Meaburn KJ, Levy N, Toniolo D, Bridger JM. Meaburn KJ, et al. Biochem Soc Trans. 2005 Dec;33(Pt 6):1438-40. doi: 10.1042/BST0331438. Biochem Soc Trans. 2005. PMID: 16246140
Cited by
- Nuclear envelope budding inhibition slows down progerin-induced aging process.
Wang X, Ma L, Lu D, Zhao G, Ren H, Lin Q, Jia M, Huang F, Wang S, Xu Z, Yang Z, Chu Y, Xu Z, Li W, Yu L, Jiang Q, Zhang C. Wang X, et al. Proc Natl Acad Sci U S A. 2024 Oct 8;121(41):e2321378121. doi: 10.1073/pnas.2321378121. Epub 2024 Oct 1. Proc Natl Acad Sci U S A. 2024. PMID: 39352925 - Emerin mislocalization during chromatin bridge resolution can drive prostate cancer cell invasiveness in a collagen-rich microenvironment.
Popęda M, Kowalski K, Wenta T, Beznoussenko GV, Rychłowski M, Mironov A, Lavagnino Z, Barozzi S, Richert J, Bertolio R, Myszczyński K, Szade J, Bieńkowski M, Miszewski K, Matuszewski M, Żaczek AJ, Braga L, Del Sal G, Bednarz-Knoll N, Maiuri P, Nastały P. Popęda M, et al. Exp Mol Med. 2024 Sep;56(9):2016-2032. doi: 10.1038/s12276-024-01308-w. Epub 2024 Sep 2. Exp Mol Med. 2024. PMID: 39218980 Free PMC article. - Emerin deficiency drives MCF7 cells to an invasive phenotype.
Hansen E, Rolling C, Wang M, Holaska JM. Hansen E, et al. Sci Rep. 2024 Aug 28;14(1):19998. doi: 10.1038/s41598-024-70752-5. Sci Rep. 2024. PMID: 39198511 Free PMC article. - PQBP3 prevents senescence by suppressing PSME3-mediated proteasomal Lamin B1 degradation.
Yoshioka Y, Huang Y, Jin X, Ngo KX, Kumaki T, Jin M, Toyoda S, Takayama S, Inotsume M, Fujita K, Homma H, Ando T, Tanaka H, Okazawa H. Yoshioka Y, et al. EMBO J. 2024 Sep;43(18):3968-3999. doi: 10.1038/s44318-024-00192-4. Epub 2024 Aug 5. EMBO J. 2024. PMID: 39103492 Free PMC article. - Role of Emerin in regulating fibroblast differentiation and migration at the substrate of stiffness coupled topology.
Yang T, Wang L, Ma H, Li K, Wang Y, Tang W, Wang Z, An M, Gao X, Xu L, Guo Y, Guo J, Liu Y, Wang H, Liu Y, Zhang Q. Yang T, et al. Acta Biochim Biophys Sin (Shanghai). 2024 Jul 8;56(9):1387-1400. doi: 10.3724/abbs.2024094. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38978507 Free PMC article.
References
- Bengtsson, L., and K.L. Wilson. 2004. Multiple and surprising new functions for emerin, a nuclear membrane protein. Curr. Opin. Cell Biol. 16:73–79. - PubMed
- Bione, S., E. Maestrini, S. Rivella, M. Mancini, S. Regis, G. Romeo, and D. Toniolo. 1994. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat. Genet. 8:323–327. - PubMed
- Bonne, G., M.R. Di Barletta, S. Varnous, H.M. Becane, E.H. Hammouda, L. Merlini, F. Muntoni, C.R. Greenberg, F. Gary, J.A. Urtizberea, et al. 1999. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat. Genet. 21:285–288. - PubMed
- Broers, J.L., E.A. Peeters, H.J. Kuijpers, J. Endert, C.V. Bouten, C.W. Oomens, F.P. Baaijens, and F.C. Ramaekers. 2004. Decreased mechanical stiffness in LMNA−/− cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum. Mol. Genet. 13:2567–2580. - PubMed
- Burke, B., and C.L. Stewart. 2002. Life at the edge: the nuclear envelope and human disease. Nat. Rev. Mol. Cell Biol. 3:575–585. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources