Stearoyl-CoA desaturase-2 gene expression is required for lipid synthesis during early skin and liver development - PubMed (original) (raw)
Stearoyl-CoA desaturase-2 gene expression is required for lipid synthesis during early skin and liver development
Makoto Miyazaki et al. Proc Natl Acad Sci U S A. 2005.
Abstract
There are four known stearoyl-CoA desaturase (SCD) enzyme isoforms in mouse and two in humans that are required for the biosynthesis of monounsaturated fatty acids, mainly oleate. SCD1 isoform plays a role in the regulation of energy metabolism and lipid synthesis, but the roles of the other SCD isoforms have not been investigated. Here we show that the SCD2 isoform is important in lipid synthesis in early development and is required for survival. SCD2-deficient (Scd2-/-) neonatal mice have a skin permeability barrier defect and a specific repartitioning of linoleic acid from epidermal acylceramide species into phospholipids. SCD2 expression is high in liver of wild-type mouse embryos and neonates between embryonic day 18.5 and 21 days of age and is decreased in adult mice. SCD1 expression, on the other hand, is induced after weaning. The liver, skin, and plasma triglyceride contents are decreased in the neonates but are not altered in the adult Scd2-/- mice. These results indicate that, although SCD1 expression is important in adult mice, SCD2 is crucial in the synthesis of monounsaturated fatty acids that are required for maintaining normal epidermal permeability barrier function and biosynthesis of lipids during early skin and liver development.
Figures
Fig. 1.
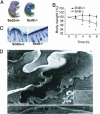
Generation of _Scd2_-/- mice. (A) Targeting strategy for disruption of the Scd2 gene. A neomycin-resistant cassette replaced the six exons of the gene by homologous recombination, resulting in the replacement of the complete coding region of the Scd2 gene. (B) PCR analysis of DNA isolated from different genotypes of offspring using appropriate primers. In breeding heterozygotes, wild-type, heterozygotes and homozygotes mice were born in Mendelian fashion. (C) SCD isoform expression in adult _Scd2_-/- mice. SCD1 expression was assessed in liver and brain, SCD3 was assessed in Harderian gland, and SCD4 expression was assessed in heart. (D) Gross appearance of newborn _Scd2_-/- mice. _Scd2_-/- neonatal mice were smaller in size with a shiny skin. (E) Adult _Scd2_-/- mice have a twisted tail.
Fig. 2.
Fetal impairment of the epidermal barrier in _Scd2_-/- mice. (A) Skin-permeability barrier assays using 0.1% toluidine blue. Mice were immersed in toluidine blue dye for 10 min, rinsed with PBS, and photographed. (B) Rapid weight loss in _Scd2_-/- mice is presumably due to water loss (*, P < 0.01, n = 6-8). (C) Light micrographs of skin of 1-day _Scd2_-/- mice (Left) in comparison with Scd2+/+ mice (Right). (D) Ultrastructure of _Scd2_-/- epidermis. (A) _Scd2_-/- mice exhibited delayed lamellar membrane maturation (double arrow) and phase separation (asterisks), forming a two-phase extracellular system. (B) Lamellar bodies in the _SCD2_-/- mice. (C) The wild-type control shows normal mature lamellar membranes in stratum corneum (single arrows). (Bar, 0.5 μm.) (A and C) Ruthenium tetroxide postfixation.
Fig. 3.
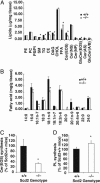
Epidermal lipid contents in _Scd2_-/- mice. Lipids were extracted from epidermis (2.5 mg), separated by TLC, and quantitated by GLC. (Chol, free cholesterol; DAG, diacylglycerols; Cer, ceramides; GluCer, glucosylceramides; PE, phosphatidylethanolamine; PC, phosphatidylcholine; PS, phosphatidylserine; PI, phophatidylinositol; and SM, sphingomyelin). (A) Quantitations of lipids. The data are presented as the mean ± SD (n = 4-5). *; P < 0.01 vs. Scd2+/+ mice. (B) Fatty acid contents in total epidermal lipids. Fatty acids were transmethylated and analyzed by GLC. n = 4∼6; *, P < 0.01. (C) Acylceramide and (D) phospholipid synthesis. The incorporation of 14C-linoleic acid into acylceramide and phospholipids was assessed in cultured epidermis. Epidermal sheets were organ-cultured for 12 h in MEM containing trace linoleic acid (5 μCi), and lipids were extracted as detailed in Materials and Methods. Data are expressed as a percentage of the values of Scd2+/+ mice. Data are presented as the mean ± SD (n = 6); *, P < 0.01. Cer(AS), acylceramides; PL, phospholipids.
Fig. 4.
Gene expression in epidermis and dermis of Scd2+/+ and _Scd2_-/- mice. (A) Full thickness skin of the newborn _Scd2_-/- and Scd2+/+ mice was removed, the s.c. fat was scraped away under a dissecting microscope, and the sample was immersed in 10 mM EDTA in PBS for 1 h at 37°C. The skin was then chilled on ice, and the epidermis was quickly peeled away from the dermis. RNA was isolated from the epidermis and dermis and analyzed by Northern blot for expression of SCD1, SCD2, acyl-CoA:diacylglycerol acyl transferase-2, Gba, and involucrin. pAL15 expression was used as loading control. (B) SCD3 mRNA levels were measured by real-time PCR. *, P < 0.05 vs. Scd2+/+ mice (n = 4∼6).
Fig. 5.
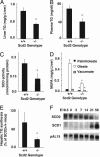
Hepatic and plasma TG levels and hepatic SCD activity in newborn mice. (A) Hepatic and (B) plasma TG contents were analyzed by GLC. (C) SCD activity was measured as conversion rate of [14C]18:0-CoA to [14C]-18:1-CoA. (D) MUFA levels in TG in liver of newborn and adult mice. Palmitoleate [16:1(_n_-7)], oleate [18:1(_n_-9)], and vaccenate [18:1(_n_-7)] contents in TG were measured by GLC. (E) Rate of TG synthesis in liver of newborn mice. Mice were i.p. injected with 10 μCi of 3H-glycerol 1 h before being killed. After extraction of hepatic lipids, TG and phospholipids were separated by TLC, and radioactivity was determined. Data are expressed as a percentage of the values of SCD2+/+ mice). *, P < 0.01 vs. Scd2+/+ mice (n = 6). (F) Developmental expression of SCD1 and -2 in mouse liver. The expression level of SCD1 and -2 in liver of mice at embryonic day 18.5, newborn, and 3, 7, 14, 21, and 56 days of age was measured by Northern blot analysis. Expression levels were obtained from pooled total RNA from four to five mice. pAL15 mRNA was used as a loading control.
Similar articles
- Identification and characterization of murine SCD4, a novel heart-specific stearoyl-CoA desaturase isoform regulated by leptin and dietary factors.
Miyazaki M, Jacobson MJ, Man WC, Cohen P, Asilmaz E, Friedman JM, Ntambi JM. Miyazaki M, et al. J Biol Chem. 2003 Sep 5;278(36):33904-11. doi: 10.1074/jbc.M304724200. Epub 2003 Jun 18. J Biol Chem. 2003. PMID: 12815040 - The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1.
Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM. Miyazaki M, et al. J Biol Chem. 2000 Sep 29;275(39):30132-8. doi: 10.1074/jbc.M005488200. J Biol Chem. 2000. PMID: 10899171 - Regulation of stearoyl-CoA desaturases and role in metabolism.
Ntambi JM, Miyazaki M. Ntambi JM, et al. Prog Lipid Res. 2004 Mar;43(2):91-104. doi: 10.1016/s0163-7827(03)00039-0. Prog Lipid Res. 2004. PMID: 14654089 Review. - Regulation of stearoyl-CoA desaturase expression.
Ntambi JM, Miyazaki M, Dobrzyn A. Ntambi JM, et al. Lipids. 2004 Nov;39(11):1061-5. doi: 10.1007/s11745-004-1331-2. Lipids. 2004. PMID: 15726820 Review.
Cited by
- A Discussion on the Relationship between Skin Lipid Metabolism and Whole-Body Glucose and Lipid Metabolism: Systematic Review.
Dumas SN, Ntambi JM. Dumas SN, et al. J Cell Signal (Los Angel). 2018;3(3):189. doi: 10.4172/2576-1471.1000189. Epub 2018 Oct 10. J Cell Signal (Los Angel). 2018. PMID: 30474082 Free PMC article. - Gene expression profiling of the developing mouse kidney and embryo.
Shaw L, Johnson PA, Kimber SJ. Shaw L, et al. In Vitro Cell Dev Biol Anim. 2010 Feb;46(2):155-65. doi: 10.1007/s11626-009-9254-x. Epub 2009 Dec 9. In Vitro Cell Dev Biol Anim. 2010. PMID: 19998061 - Lack of hexose-6-phosphate dehydrogenase impairs lipid mobilization from mouse adipose tissue.
Bujalska IJ, Hewitt KN, Hauton D, Lavery GG, Tomlinson JW, Walker EA, Stewart PM. Bujalska IJ, et al. Endocrinology. 2008 May;149(5):2584-91. doi: 10.1210/en.2007-1705. Epub 2008 Jan 24. Endocrinology. 2008. PMID: 18218694 Free PMC article. - Toll-Like Receptors Induce Signal-Specific Reprogramming of the Macrophage Lipidome.
Hsieh WY, Zhou QD, York AG, Williams KJ, Scumpia PO, Kronenberger EB, Hoi XP, Su B, Chi X, Bui VL, Khialeeva E, Kaplan A, Son YM, Divakaruni AS, Sun J, Smale ST, Flavell RA, Bensinger SJ. Hsieh WY, et al. Cell Metab. 2020 Jul 7;32(1):128-143.e5. doi: 10.1016/j.cmet.2020.05.003. Epub 2020 Jun 8. Cell Metab. 2020. PMID: 32516576 Free PMC article. - Stearoyl-CoA Desaturase Promotes Liver Fibrosis and Tumor Development in Mice via a Wnt Positive-Signaling Loop by Stabilization of Low-Density Lipoprotein-Receptor-Related Proteins 5 and 6.
Lai KKY, Kweon SM, Chi F, Hwang E, Kabe Y, Higashiyama R, Qin L, Yan R, Wu RP, Lai K, Fujii N, French S, Xu J, Wang JY, Murali R, Mishra L, Lee JS, Ntambi JM, Tsukamoto H. Lai KKY, et al. Gastroenterology. 2017 May;152(6):1477-1491. doi: 10.1053/j.gastro.2017.01.021. Epub 2017 Jan 29. Gastroenterology. 2017. PMID: 28143772 Free PMC article.
References
- Enoch, H. G., Catala, A. & Strittmatter, P. (1976) J. Biol. Chem. 251, 5095-5103. - PubMed
- Miyazaki, M. & Ntambi, J. M. (2003) Prostaglandins Leukotrienes Essent. Fatty Acids 68, 113-121. - PubMed
- Kaestner, K. H., Ntambi, J. M., Kelly, T. J., Jr., & Lane, M. D. (1989) J. Biol. Chem. 264, 14755-14761. - PubMed
- Miyazaki, M., Jacobson, M. J., Man, W. C., Cohen, P., Asilmaz, E., Friedman, J. M. & Ntambi, J. M. (2003) J. Biol. Chem. 278, 33904-33911. - PubMed
- Ntambi, J. M., Buhrow, S. A., Kaestner, K. H., Christy, R. J., Sibley, E., Kelly, T. J., Jr., & Lane, M. D. (1988) J. Biol. Chem. 263, 17291-17300. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials