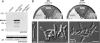Myosin-V, Kinesin-1, and Kinesin-3 cooperate in hyphal growth of the fungus Ustilago maydis - PubMed (original) (raw)
Myosin-V, Kinesin-1, and Kinesin-3 cooperate in hyphal growth of the fungus Ustilago maydis
Isabel Schuchardt et al. Mol Biol Cell. 2005 Nov.
Abstract
Long-distance transport is crucial for polar-growing cells, such as neurons and fungal hyphae. Kinesins and myosins participate in this process, but their functional interplay is poorly understood. Here, we investigate the role of kinesin motors in hyphal growth of the plant pathogen Ustilago maydis. Although the microtubule plus-ends are directed to the hyphal tip, of all 10 kinesins analyzed, only conventional kinesin (Kinesin-1) and Unc104/Kif1A-like kinesin (Kinesin-3) were up-regulated in hyphae and they are essential for extended hyphal growth. deltakin1 and deltakin3 mutant hyphae grew irregular and remained short, but they were still able to grow polarized. No additional phenotype was detected in deltakin1rkin3 double mutants, but polarity was lost in deltamyo5rkin1 and deltamyo5rkin3 mutant cells, suggesting that kinesins and class V myosin cooperate in hyphal growth. Consistent with such a role in secretion, fusion proteins of green fluorescent protein and Kinesin-1, Myosin-V, and Kinesin-3 accumulate in the apex of hyphae, a region where secretory vesicles cluster to form the fungal Spitzenkörper. Quantitative assays revealed a role of Kin3 in secretion of acid phosphatase, whereas Kin1 was not involved. Our data demonstrate that just two kinesins and at least one myosin support hyphal growth.
Figures
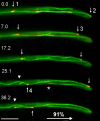
Figure 1.
Orientation of MT plus-ends in hyphae of U. maydis. A fusion of RFP and Peb1, an EB1-homologue that localizes to growing MT plus-ends (Straube et al., 2003) marks microtubule ends that grow toward the hyphal apex, indicating that kinesins could support polar hyphal growth. Arrows indicate four MT tips; arrowhead marks a microtubule that elongates toward the proximal end of the tip cell. Note that microtubules are not connected to the spindle pole body (asterisk marks minus-end of a cytoplasmic microtubule; the corresponding plus end is marked by “2” in first frame). Time in seconds is given. Bar, 5 μm.
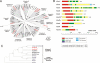
Figure 2.
Sequence analysis of kinesins in U. maydis. (A) The genome of U. maydis encodes for 10 kinesins. Nine of these kinesin motors belong to defined subfamilies, whereas UmKin9 is ungrouped. The tree is based on an alignment of the motor domain of fungal and animal kinesins. Cn, Cryptococcus neoformans; Um, U. maydis; Hs, Homo sapiens; Mm, Mus musculus; Dm, Drosophila melanogaster; Sp, Strongylocentrotus purpuratus; Spo, S. pombe; Sc, S. cervisiae; Ca, Candida albicans; Nc, Neurospora crasssa; An/AN, A. nidulans. Note that only selected kinesins from animal and fungal species were included. Accession numbers are given in supplementary files. Closed circle, >80% bootstrap; open circle, 60–80% bootstrap. (B) Domain organization of all kinesins in U. maydis. Domain analysis was done using The SMART and PROSITE server. Note that the domain organization is based on sequence prediction and comparison with kinesin motors from other organisms. (C) Tree of members of the Kinesin-7 family. Kinesin-7 from ascomycete fungi group together, which corresponds with a role in cell polarity (Browning et al., 2000) and hyphal growth (Konzack et al., 2004). In contrast, representatives from basidiomycete fungi are more closely related to CENP-E motors from animals, where they participate in chromosome inheritance (Yen et al., 1991). For species names see Fig. 2A. Accession numbers are given in supplemental files. The tree is based on a comparison of motor domains. Bootstrap values are given in percentage.
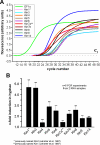
Figure 3.
Real-time PCR analysis of expression of kinesins in U. maydis. (A) Graph showing fluorescent intensities relative to PCR cycles, by using complete RNA from the filamentously growing strain AB33, specific primers for all kinesins (Kin1–Kin14) and the housekeeping gene EF1-α. Amplification increases after 20–28 cycles indicating that all genes are expressed. C_t_: threshold cycle above background indicating beginning of logarithmic amplification. (B) Comparison of the expression of all kinesins in filamentously growing AB33 and yeast-like control strain AB34 under the same growth conditions demonstrate that kin1 and kin3 are up-regulated in hyphal growth. This corresponds well with the important role of these kinesins in filamentous growth.
Figure 4.
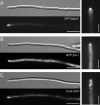
Morphology phenotype of kinesin null mutants. (A) Most kinesin null mutants neither have defects in the yeast-like stage (our unpublished data) nor show impaired hyphal growth. Note that most mutants are in the ABB33 background (see Materials and Methods), whereas Δ_kin14_ hyphae were obtained from crossing compatible mutant strains on charcoal plates. All bars, 10 μm. (B) Δ_kin1_ and Δ_kin3_ mutant hyphae often grow in a bipolar manner and are unable to form long filaments. No additional defects were seen in Δ_kin1_r_kin3_ double mutants. Note that, for unknown reason, the double mutants again formed vacuolated hyphal parts. Bar, 10 μm. (C) Treatment of AB33 hyphae with 10 μM benomyl for 12 h resulted in short hyphae that showed phenotypic similarities to Δ_kin1_ and Δ_kin3_ mutant hyphae. Bar, 10 μm. (D) GFP-labeled MTs were normal in Δ_kin1_ and Δ_kin3_ mutants (see Steinberg et al., 2001 for MT organization in wild-type cells), which demonstrates that the morphological phenotype of the kinesin mutants is not due to a global defect in MT organization. Bar, 10 μm.
Figure 5.
Effect of high levels of Kin1 on the phenotype of a class V myosin mutant. (A) An affinity-purified rabbit antibody raised against peptides in the motor domain and the tail of Kin1 recognizes a faint band of ∼110 kDa in extracts of myo5 null mutants (control). Expression of Kin1 under the control of the crg_-promoter in the presence of arabinose shows strongly induced expression (Δmyo5rKin1, ON), but all expression of kin1 was repressed in CM-G (Δmyo5rKin1, OFF). (B) On CM-A plates (ON, arabinose), wild-type cells form colonies after 2 d at 28°C (control), whereas plate growth of Δ_myo5 mutant cells was heavily impaired (Δmyo5). High levels of Kin1 did not rescue this phenotype (Δmyo5 + Kin1↑). (C) Yeast-like control cells form elongated “cigar-shaped” cells that grow by polar budding (con). Deletion of myo5 led to severe defects in polar growth and aggregates of cells that still have a tendency to grow in a polar manner (Weber et al., 2003). In CM-A medium, high levels of Kin1 did not rescue the Δ_myo5_ phenotype (Δmyo5 + Kin1↑). Bar, 10 μm.
Figure 6.
Phenotype of conditional Δ_myo5_r_kin1_ and Δ_myo5_r_kin3_ mutants. (A) Although deletion of Δ_myo5_ mutants impaired hyphal morphology, cells of strain AB33ΔMyo5 are still able to grow polarized. Bar, 10 μm. (B) Hyphal polarity is lost in the absence of MTs after overnight treatment with 10 μM benomyl, indicating that both F-actin and MTs are needed for polar hyphal growth. Bar, 10 μm. (C) Hyphal growth of Δ_myo5_r_kin1_ double mutants (strain AB33ΔMyo5rKin3) was induced for ∼16 h by growing cells in NM-G. This medium also repressed kin1 (see E) that is placed under the control of the crg promoter. In the absence of Kin1, myo5 null mutant lost their ability to form hyphae, indicating that both Myo5 and Kin1 activity is needed to maintain polarized hyphal growth. Bar, 10 μm. (D) In similar experiments, filamentous growth was induced, whereas kin3 was repressed in Δ_myo5_r_kin3_ mutants (strain AB33ΔMyo5rKin3; see below, E). In the absence of Kin3 polarized hyphal growth was also almost abolished, arguing that Kin3 also cooperates with Myo5 to maintain tip growth. Bar, 10 μm. (E) Western blots showing that Kin1 and Kin3 expression is repressed in CM-G (glu, OFF), whereas high expression levels were found in CM-A (ara, ON).
Figure 7.
Secretion and localization defects in Δ_kin1_ and Δ_myo5_ mutants. (A) A fusion protein of a triple GFP-tag fused to Myo5 localizes to the hyphal apex. Note that the fusion protein localizes to a focused dot at the hyphal tip, where the Spitzenkörper is located (Lehmler et al., 1997). Bars, 10 and 3 μm. (B) A fusion protein of a triple GFP-tag fused to Kin1 also localizes to the hyphal apex, but the signal is often more dispersed and a strong cytoplasmic background was seen. Bar, 10 and 3 μm. (C) A fusion protein of a GFP-tag fused to Kin3 localized as rapidly moving dots along the length of the hyphae but also concentrates near the hyphal tip. Note that the apical Kin3-GFP does not reach the apex and is more dispersed. Bar, 10 and 3 μm.
Figure 8.
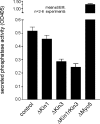
Quantitative analysis of acid phosphatase secretion in hyphae of U. maydis. For analysis of the extracellular activity of the marker enzyme acid phosphatase, cells were incubated in phosphate-free medium and the release of nitrophenon from p_-nitrophenyl phosphate was measured as an indication of secreted enzyme activity. Δ_kin1 mutants were not significantly impaired in secretion of acid phosphatase, whereas secretion was found to be reduced in Δ_kin3_ mutants. Consistently, Δ_kin1_r_kin3_ double mutants show no additional phenotype. For unknown reasons, deletion of myo5 even increased secretion, a phenomenon that was also found in Δ_myo5_r_kin1_ mutants (our unpublished data).
Figure 9.
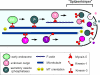
Model on the role of Kin1, Kin3, and Myo5 in polar fungal growth. Polar hyphal growth is supported by long-distance delivery of growth supplies to the expanding hyphal tip. MT plus-ends are directed to the growing tip, and Kinesin-1, Kinesin-3, and Myosin-V use cytoskeletal tracks to deliver their cargo to the growth region. The ability to grow polarized is only abolished when both Myosin-V and either Kinesin-1 or Kinesin-3 are blocked. Kinesin-3 most likely transports acid phosphatase containing vesicles, as well as early endosomes (Wedlich-Söldner et al., 2002), whereas the putative exocytic cargo of Kinesin-1 and Myosin-V is unknown. In the hyphal apex, secretory vesicles and attached motors cluster to form the fungal Spitzenkörper that is thought to mediate controlled local exocytosis and thereby determines tip growth.
Similar articles
- A balance of KIF1A-like kinesin and dynein organizes early endosomes in the fungus Ustilago maydis.
Wedlich-Söldner R, Straube A, Friedrich MW, Steinberg G. Wedlich-Söldner R, et al. EMBO J. 2002 Jun 17;21(12):2946-57. doi: 10.1093/emboj/cdf296. EMBO J. 2002. PMID: 12065408 Free PMC article. - Aspergillus myosin-V supports polarized growth in the absence of microtubule-based transport.
Zhang J, Tan K, Wu X, Chen G, Sun J, Reck-Peterson SL, Hammer JA 3rd, Xiang X. Zhang J, et al. PLoS One. 2011;6(12):e28575. doi: 10.1371/journal.pone.0028575. Epub 2011 Dec 14. PLoS One. 2011. PMID: 22194856 Free PMC article. - The RNA-binding protein Rrm4 is essential for polarity in Ustilago maydis and shuttles along microtubules.
Becht P, König J, Feldbrügge M. Becht P, et al. J Cell Sci. 2006 Dec 1;119(Pt 23):4964-73. doi: 10.1242/jcs.03287. Epub 2006 Nov 14. J Cell Sci. 2006. PMID: 17105762 - The role of microtubules in cellular organization and endocytosis in the plant pathogen Ustilago maydis.
Steinberg G, Fuchs U. Steinberg G, et al. J Microsc. 2004 May;214(Pt 2):114-23. doi: 10.1111/j.0022-2720.2004.01319.x. J Microsc. 2004. PMID: 15102060 Review. - Tracks for traffic: microtubules in the plant pathogen Ustilago maydis.
Steinberg G. Steinberg G. New Phytol. 2007;174(4):721-733. doi: 10.1111/j.1469-8137.2007.02072.x. New Phytol. 2007. PMID: 17504456 Review.
Cited by
- Molecular Mechanisms Involved in the Multicellular Growth of Ustilaginomycetes.
Martínez-Soto D, Ortiz-Castellanos L, Robledo-Briones M, León-Ramírez CG. Martínez-Soto D, et al. Microorganisms. 2020 Jul 18;8(7):1072. doi: 10.3390/microorganisms8071072. Microorganisms. 2020. PMID: 32708448 Free PMC article. Review. - Neurospora crassa NKIN2, a kinesin-3 motor, transports early endosomes and is required for polarized growth.
Seidel C, Moreno-Velásquez SD, Riquelme M, Fischer R. Seidel C, et al. Eukaryot Cell. 2013 Jul;12(7):1020-32. doi: 10.1128/EC.00081-13. Epub 2013 May 17. Eukaryot Cell. 2013. PMID: 23687116 Free PMC article. - Hyphal growth: a tale of motors, lipids, and the Spitzenkörper.
Steinberg G. Steinberg G. Eukaryot Cell. 2007 Mar;6(3):351-60. doi: 10.1128/EC.00381-06. Epub 2007 Jan 26. Eukaryot Cell. 2007. PMID: 17259546 Free PMC article. Review. No abstract available. - Interaction with mycorrhiza helper bacterium Streptomyces sp. AcH 505 modifies organisation of actin cytoskeleton in the ectomycorrhizal fungus Amanita muscaria (fly agaric).
Schrey SD, Salo V, Raudaskoski M, Hampp R, Nehls U, Tarkka MT. Schrey SD, et al. Curr Genet. 2007 Aug;52(2):77-85. doi: 10.1007/s00294-007-0138-x. Epub 2007 Jul 14. Curr Genet. 2007. PMID: 17632722 - The machinery for cell polarity, cell morphogenesis, and the cytoskeleton in the Basidiomycete fungus Ustilago maydis-a survey of the genome sequence.
Banuett F, Quintanilla RH Jr, Reynaga-Peña CG. Banuett F, et al. Fungal Genet Biol. 2008 Aug;45 Suppl 1(Suppl 1):S3-S14. doi: 10.1016/j.fgb.2008.05.012. Epub 2008 Jun 7. Fungal Genet Biol. 2008. PMID: 18582586 Free PMC article. Review.
References
- Banks, G. R., Shelton, P. A., Kanuga, N., Holden, D. W., and Spanos, A. (1993). The Ustilago maydis nar1 gene encoding nitrate reductase activity: sequence and transcriptional regulation. Gene 131, 69–78. - PubMed
- Bartnicki-Garcia, S., Bartnicki, D. D., Gierz, G., Lopez-Franco, R., and Bracker, C. E. (1995). Evidence that Spitzenkorper behavior determines the shape of a fungal hypha: a test of the hyphoid model. Exp. Mycol. 19, 153–159. - PubMed
- Bottin, A., Kamper, J., and Kahmann, R. (1996). Isolation of a carbon source-regulated gene from Ustilago maydis. Mol. Gen. Genet. 253, 342–352. - PubMed
- Brachmann, A., König, J., Julius, C., and Feldbrügge, M. (2004). A reverse genetic approach for generating gene replacement mutants in Ustilago maydis. Mol. Genet. Genomics 272, 488. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous