Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2 - PubMed (original) (raw)
. 2005 Aug 25;436(7054):1166-73.
doi: 10.1038/nature03897.
Delphine Gobert, Heather Harding, Barbara Herdy, Mounia Azzi, Martin Bruno, Michael Bidinosti, Cyrinne Ben Mamou, Edwige Marcinkiewicz, Madoka Yoshida, Hiroaki Imataka, A Claudio Cuello, Nabil Seidah, Wayne Sossin, Jean-Claude Lacaille, David Ron, Karim Nader, Nahum Sonenberg
Affiliations
- PMID: 16121183
- PMCID: PMC1464117
- DOI: 10.1038/nature03897
Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2
Mauro Costa-Mattioli et al. Nature. 2005.
Abstract
Studies on various forms of synaptic plasticity have shown a link between messenger RNA translation, learning and memory. Like memory, synaptic plasticity includes an early phase that depends on modification of pre-existing proteins, and a late phase that requires transcription and synthesis of new proteins. Activation of postsynaptic targets seems to trigger the transcription of plasticity-related genes. The new mRNAs are either translated in the soma or transported to synapses before translation. GCN2, a key protein kinase, regulates the initiation of translation. Here we report a unique feature of hippocampal slices from GCN2(-/-) mice: in CA1, a single 100-Hz train induces a strong and sustained long-term potentiation (late LTP or L-LTP), which is dependent on transcription and translation. In contrast, stimulation that elicits L-LTP in wild-type slices, such as four 100-Hz trains or forskolin, fails to evoke L-LTP in GCN2(-/-) slices. This aberrant synaptic plasticity is mirrored in the behaviour of GCN2(-/-) mice in the Morris water maze: after weak training, their spatial memory is enhanced, but it is impaired after more intense training. Activated GCN2 stimulates mRNA translation of ATF4, an antagonist of cyclic-AMP-response-element-binding protein (CREB). Thus, in the hippocampus of GCN2(-/-) mice, the expression of ATF4 is reduced and CREB activity is increased. Our study provides genetic, physiological, behavioural and molecular evidence that GCN2 regulates synaptic plasticity, as well as learning and memory, through modulation of the ATF4/CREB pathway.
Figures
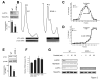
Figure 1
Unusual properties of LTP induced in slices from GCN2 -/- mice. A) One train (100 Hz for 1s) of high-frequency stimulation (HFS) elicited E-LTP in WT slices but produced a robust and L-LTP in GCN2 -/- slices. B) Sustained LTP in GCN2 -/- slices is reduced by anisomycin (ANISO, 40μM), actinomycin-D (ACTD, 40μM) or the PKA inhibitor KT5720 (1μM). C) The enhanced LTP in GCN2 -/- slices is reduced by actinomycin-D, at later time points (>90 min) when applied 15 min prior to and 45 min post-tetanus. D) L-LTP induced by four 100 Hz trains at 5 min intervals is stable in WT slices but not in GCN2 -/- slices.
Figure 2
ATF4 mRNA translation is downregulated in GCN2 -/- mice. A) Western blots performed on hippocampal extracts show that eIF2α phosphorylation is reduced in GCN2 -/- (n=3) as compared to WT mice (n=3). B) In polysome profiles from hippocampal extracts, ATF4 mRNA is in lighter fractions in GCN2 -/- (left panel) than in WT (right panel) controls as determined by RT-PCR analysis. C) Quantification of the band intensities in each fraction from ATF4 mRNA, panel B. D) for data in B, band intensities are quantified for each fraction of β-actin mRNA. E) In pooled hippocampal extracts, expression of ATF4 is reduced in GCN2 -/- mice. F) Real-time RT-PCR analysis reveals increased expression of CREB-dependent genes in hippocampal extracts from GCN2 -/- vs. WT mice (for both, n=5); mRNA expression is given as % of controls; *p < 0.05, **p < 0.01. G) Forskolin decreases GCN2 and eIF2α phosphorylation. In immunoblots of homogenates of CA1 region (from slices frozen immediately after tetanic stimulation), phosphorylated GCN2 (top) and eIF2α (middle panel) are decreased five minutes after forskolin application.
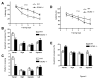
Figure 3
GCN2 -/- mice are impaired in contextual but not auditory fear conditioning. A) Though acquisition of contextual freezing (with two pairings) is similar in GCN2 -/- (filled squares, n=10) and WT (open squares, n=12) mice, contextual freezing, measured during 2 min period before first shock (pre-shocks) and 1 min period after last shock (post-shocks), is impaired in GCN2 -/- mice 1 and 10 days after acquisition. B) Auditory fear conditioning is intact in freezing to tone over two pairings and in long term memory tests at 1 and 10 days later in GCN2 -/- mice. Labels indicate genotype and whether freezing was to tone (CS) or during 2 min before tone (pre-CS) (Means ± SEM).
Figure 4
Long-term spatial memory of GCN2 -/- mice is enhanced after weak training but impaired after more intense training (in the Morris water maze) A) Escape latencies in hidden platform tests (3 trial per day), plotted as function of training days (WT, open squares, n = 16 and GCN2 -/-, filled squares, n = 15), are shorter for WT than GCN2 -/- mice. B) After completion of training, WT mice show preferential quadrant occupancy. C) WT mice crossed the previous site where the platform was located more times than GCN2 -/- mice (p < 0.001). D) When locating hidden platform (1 trial per day), escape latencies were consistently shorter for GCN2 -/- mice than for WT (for both, n=15). E) In occupancy test, GCN2 -/- mice spent more time in the trained quadrant (Means ± SEM).
Similar articles
- Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage.
Wood MA, Kaplan MP, Park A, Blanchard EJ, Oliveira AM, Lombardi TL, Abel T. Wood MA, et al. Learn Mem. 2005 Mar-Apr;12(2):111-9. doi: 10.1101/lm.86605. Learn Mem. 2005. PMID: 15805310 Free PMC article. - Selective modulation of some forms of schaffer collateral-CA1 synaptic plasticity in mice with a disruption of the CPEB-1 gene.
Alarcon JM, Hodgman R, Theis M, Huang YS, Kandel ER, Richter JD. Alarcon JM, et al. Learn Mem. 2004 May-Jun;11(3):318-27. doi: 10.1101/lm.72704. Learn Mem. 2004. PMID: 15169862 Free PMC article. - A form of long-lasting, learning-related synaptic plasticity in the hippocampus induced by heterosynaptic low-frequency pairing.
Huang YY, Pittenger C, Kandel ER. Huang YY, et al. Proc Natl Acad Sci U S A. 2004 Jan 20;101(3):859-64. doi: 10.1073/pnas.2237201100. Epub 2004 Jan 7. Proc Natl Acad Sci U S A. 2004. PMID: 14711997 Free PMC article. - BDNF-induced local protein synthesis and synaptic plasticity.
Leal G, Comprido D, Duarte CB. Leal G, et al. Neuropharmacology. 2014 Jan;76 Pt C:639-56. doi: 10.1016/j.neuropharm.2013.04.005. Epub 2013 Apr 16. Neuropharmacology. 2014. PMID: 23602987 Review. - Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases.
Nguyen PV, Woo NH. Nguyen PV, et al. Prog Neurobiol. 2003 Dec;71(6):401-37. doi: 10.1016/j.pneurobio.2003.12.003. Prog Neurobiol. 2003. PMID: 15013227 Review.
Cited by
- Pharmacological brake-release of mRNA translation enhances cognitive memory.
Sidrauski C, Acosta-Alvear D, Khoutorsky A, Vedantham P, Hearn BR, Li H, Gamache K, Gallagher CM, Ang KK, Wilson C, Okreglak V, Ashkenazi A, Hann B, Nader K, Arkin MR, Renslo AR, Sonenberg N, Walter P. Sidrauski C, et al. Elife. 2013 May 28;2:e00498. doi: 10.7554/eLife.00498. Elife. 2013. PMID: 23741617 Free PMC article. - Calmodulin Triggers Activity-Dependent rRNA Biogenesis via Interaction with DDX21.
Yang JL, Sun X, Shi JX, Cui QX, Cao XY, Wang KT, An MX, Wu SJ, Yang YL, Sun HZ, Zhao WD. Yang JL, et al. J Neurosci. 2024 Aug 28;44(35):e1841232024. doi: 10.1523/JNEUROSCI.1841-23.2024. J Neurosci. 2024. PMID: 39060175 - IMPACT is a developmentally regulated protein in neurons that opposes the eukaryotic initiation factor 2α kinase GCN2 in the modulation of neurite outgrowth.
Roffé M, Hajj GN, Azevedo HF, Alves VS, Castilho BA. Roffé M, et al. J Biol Chem. 2013 Apr 12;288(15):10860-9. doi: 10.1074/jbc.M113.461970. Epub 2013 Feb 27. J Biol Chem. 2013. PMID: 23447528 Free PMC article. - General Control Non-derepressible 2 Alleviates Cartilage Degeneration and Inhibits NLRP3 Inflammasome Activation in Osteoarthritis.
Fang L, Wang Z, Liu J, Lin Y, Hao W. Fang L, et al. J Histochem Cytochem. 2024 Feb;72(2):95-108. doi: 10.1369/00221554231225514. Epub 2024 Jan 11. J Histochem Cytochem. 2024. PMID: 38213081 - Upstream Open Reading Frames Located in the Leader of Protein Kinase Mζ mRNA Regulate Its Translation.
Bal NV, Susorov D, Chesnokova E, Kasianov A, Mikhailova T, Alkalaeva E, Balaban PM, Kolosov P. Bal NV, et al. Front Mol Neurosci. 2016 Oct 13;9:103. doi: 10.3389/fnmol.2016.00103. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27790092 Free PMC article.
References
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–8. - PubMed
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–51. - PubMed
- Mathews MB, Sonenberg N, Hershey JWB. In: Translational Control of Gene Expression. Sonenberg N, Hershey JWB, Merrick WC, editors. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2000. pp. 1–33.
- Hinnebusch AG. In: Translational Control of Gene Expression. Sonenberg N, Hershey JWB, Merrick WC, editors. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2000. pp. 185–244.
- Sonenberg N, Dever TE. Eukaryotic translation initiation factors and regulators. Curr Opin Struct Biol. 2003;13:56–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous