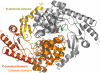Crystal structure of 5-aminolevulinate synthase, the first enzyme of heme biosynthesis, and its link to XLSA in humans - PubMed (original) (raw)
Crystal structure of 5-aminolevulinate synthase, the first enzyme of heme biosynthesis, and its link to XLSA in humans
Isabel Astner et al. EMBO J. 2005.
Abstract
5-Aminolevulinate synthase (ALAS) is the first and rate-limiting enzyme of heme biosynthesis in humans, animals, other non-plant eukaryotes, and alpha-proteobacteria. It catalyzes the synthesis of 5-aminolevulinic acid, the first common precursor of all tetrapyrroles, from glycine and succinyl-coenzyme A (sCoA) in a pyridoxal 5'-phosphate (PLP)-dependent manner. X-linked sideroblastic anemias (XLSAs), a group of severe disorders in humans characterized by inadequate formation of heme in erythroblast mitochondria, are caused by mutations in the gene for erythroid eALAS, one of two human genes for ALAS. We present the first crystal structure of homodimeric ALAS from Rhodobacter capsulatus (ALAS(Rc)) binding its cofactor PLP. We, furthermore, present structures of ALAS(Rc) in complex with the substrates glycine or sCoA. The sequence identity of ALAS from R. capsulatus and human eALAS is 49%. XLSA-causing mutations may thus be mapped, revealing the molecular basis of XLSA in humans. Mutations are found to obstruct substrate binding, disrupt the dimer interface, or hamper the correct folding. The structure of ALAS completes the structural analysis of enzymes in heme biosynthesis.
Figures
Figure 1
The structure of the ALAS homodimer from R. capsulatus (ALAS_Rc_) in ribbon representation. The N-terminal domain is rendered in yellow, the catalytic domain in orange, and the C-terminal domain in red. The cofactor PLP (green) is depicted in ball-and-stick representation, carbon in green, oxygen in red, nitrogen in blue, and phosphorus in magenta. This figure and most others were produced using P
Y
M
OL
(DeLano, 2002).
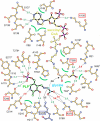
Figure 2
Schematic representation of the active site. Glycine-bound PLP and sCoA are highlighted by bonds in black; bonds of surrounding residues are shown in dark orange. Dotted lines indicate hydrogen bonds (green numbers) and salt bridges (red) between substrates or cofactors and ALAS; green semicircles indicate hydrophobic interactions. An asterisk marks residues from the second monomer. Lys248, involved in PLP binding and catalysis, is marked by a blue box, residues affected by mutations in human eALAS by a red box.
Figure 3
Detailed views of cofactor PLP and substrates in combination with their electron densities. (A) Electron density of the internal aldimine consisting of PLP (green) covalently bound to Lys248. Only residues in contact with PLP are shown. Residues of the second subunit are marked by an asterisk. (B) Electron density of PLP–glycine intermediate (cyan). The view is rotated compared to (A) to indicate the residues involved in glycine binding. Arg374 appears vital for glycine recognition. The interaction is, however, weakened by covalent bond formation—possibly aiding later decarboxylation. (C–E) The binding of sCoA to ALAS: (C) In three of four monomers of the sCoA/ALAS complex, PLP is not covalently bound to Lys248. This is documented by positive difference electron density in the absence of PLP and side chain of Lys248 (green, contoured at 3σ), the negative difference density in ‘enforced' Schiff-base bond (red, contoured at −3σ, structure in narrow bonds), and the refined electron density of unbound PLP (blue, contoured at 1σ, thick bonds). (D) The adenine and ribose moieties of sCoA bind in a hydrophobic (white) pocket with positively charged rim (blue) adjacent to the active-site channel. (E) The electron density is well defined for the 3′-phosphate ADP moiety and for the carboxylate group of sCoA, indicating that the central portion is not rigidly bound in the absence of the second substrate glycine.
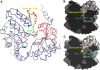
Figure 4
Open and closed conformations in ALAS. (A) Monomers A (blue) and B (gray) are overall quite similar, except for the loops β11–α14 and α14–β12 that adopt a closed conformation in monomer A (red). The open conformation may aid entry of sCoA to the active site, while the closed conformation clamps it into position prior to catalysis. In the second dimer in the asymmetric unit (and in all monomers of the substrate-bound complexes) only the closed conformation is observed. (B, C) The molecular surface of the ALAS dimer, showing a cross section through the active site in the closed (B) and in the open (C) conformation. The complete set of substrates and cofactors are included in (B) and (C) to indicate good fit in the closed conformation, allowing for high substrate specificity.
Figure 5
XLSA-causing mutations in human eALAS. (A) Sequence alignment of ALAS_Rc_ and human eALAS. Conserved residues are shown in black, all others in gray. Rectangles and arrows (colors as in Figure 1) above the alignment indicate α-helices and β-strands, respectively. Colored spheres highlight residues replaced in XLSA-causing mutations. Magenta—cofactor and substrate binding; green—mutations affecting cofactor and substrate binding indirectly; orange—mutations in the hydrophobic core; blue—surface-exposed mutation sites. Colored boxes indicate residues directly involved in substrate/cofactor binding (see Figure 2)—green, cyan and yellow indicate residues involved in PLP, glycine, and sCoA binding. (B) A model of human eALAS. Residues replaced in XLSA mutants are indicated by colored spheres. Color-coding for spheres and domains as described in (A). Pale colors indicate the second monomer.
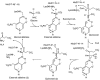
Figure 6
The reaction mechanism of ALAS. In the substrate-free state, the cofactor PLP is bound by Lys248 (ALAS_Rc_), the internal aldimine. Incoming glycine induces transaldimination, leading to PLP binding glycine rather than Lys248 (external aldimine). The substrate sCoA is nucleophilically attacked by the PLP-activated glycine, leading to the addition of succinic acid to glycine and concomitant loss of CoA. Decarboxylation (carboxylate of the glycine moiety) of this intermediate yields the PLP-bound product, released in the rate-limiting step of the reaction.
Similar articles
- Structure of the Mitochondrial Aminolevulinic Acid Synthase, a Key Heme Biosynthetic Enzyme.
Brown BL, Kardon JR, Sauer RT, Baker TA. Brown BL, et al. Structure. 2018 Apr 3;26(4):580-589.e4. doi: 10.1016/j.str.2018.02.012. Epub 2018 Mar 15. Structure. 2018. PMID: 29551290 Free PMC article. - Anti-Correlation between the Dynamics of the Active Site Loop and C-Terminal Tail in Relation to the Homodimer Asymmetry of the Mouse Erythroid 5-Aminolevulinate Synthase.
Na I, Catena D, Kong MJ, Ferreira GC, Uversky VN. Na I, et al. Int J Mol Sci. 2018 Jun 28;19(7):1899. doi: 10.3390/ijms19071899. Int J Mol Sci. 2018. PMID: 29958424 Free PMC article. - 5-aminolevulinate synthase: catalysis of the first step of heme biosynthesis.
Hunter GA, Ferreira GC. Hunter GA, et al. Cell Mol Biol (Noisy-le-grand). 2009 Feb 16;55(1):102-10. Cell Mol Biol (Noisy-le-grand). 2009. PMID: 19268008 Free PMC article. Review. - Aminolaevulinic acid synthase of Rhodobacter capsulatus: high-resolution kinetic investigation of the structural basis for substrate binding and catalysis.
Kaufholz AL, Hunter GA, Ferreira GC, Lendrihas T, Hering V, Layer G, Jahn M, Jahn D. Kaufholz AL, et al. Biochem J. 2013 Apr 15;451(2):205-16. doi: 10.1042/BJ20121041. Biochem J. 2013. PMID: 23363548 - Regulation and tissue-specific expression of δ-aminolevulinic acid synthases in non-syndromic sideroblastic anemias and porphyrias.
Peoc'h K, Nicolas G, Schmitt C, Mirmiran A, Daher R, Lefebvre T, Gouya L, Karim Z, Puy H. Peoc'h K, et al. Mol Genet Metab. 2019 Nov;128(3):190-197. doi: 10.1016/j.ymgme.2019.01.015. Epub 2019 Jan 23. Mol Genet Metab. 2019. PMID: 30737140 Review.
Cited by
- A novel hemizygous I418S mutation in the ALAS2 gene in a young Korean man with X-linked sideroblastic anemia.
Moon SY, Jun IJ, Kim JE, Lee SJ, Kim HK, Yoon SS. Moon SY, et al. Ann Lab Med. 2014 Mar;34(2):159-62. doi: 10.3343/alm.2014.34.2.159. Epub 2014 Feb 13. Ann Lab Med. 2014. PMID: 24624355 Free PMC article. No abstract available. - In Silico Comparative Transcriptome Analysis of Two Color Morphs of the Common Coral Trout (Plectropomus Leopardus).
Wang L, Yu C, Guo L, Lin H, Meng Z. Wang L, et al. PLoS One. 2015 Dec 29;10(12):e0145868. doi: 10.1371/journal.pone.0145868. eCollection 2015. PLoS One. 2015. PMID: 26713756 Free PMC article. - One ring to rule them all: trafficking of heme and heme synthesis intermediates in the metazoans.
Hamza I, Dailey HA. Hamza I, et al. Biochim Biophys Acta. 2012 Sep;1823(9):1617-32. doi: 10.1016/j.bbamcr.2012.04.009. Epub 2012 May 8. Biochim Biophys Acta. 2012. PMID: 22575458 Free PMC article. Review. - Topological and functional characterization of the ssSPTs, small activating subunits of serine palmitoyltransferase.
Harmon JM, Bacikova D, Gable K, Gupta SD, Han G, Sengupta N, Somashekarappa N, Dunn TM. Harmon JM, et al. J Biol Chem. 2013 Apr 5;288(14):10144-10153. doi: 10.1074/jbc.M113.451526. Epub 2013 Feb 20. J Biol Chem. 2013. PMID: 23426370 Free PMC article. - Case report: An infant boy with X-linked sideroblastic anaemia successfully treated by umbilical cord blood haematopoietic stem cell transplantation.
Ma Z, Li D, Yang X, Liang J, Zhu Y. Ma Z, et al. Front Genet. 2022 Nov 15;13:1009988. doi: 10.3389/fgene.2022.1009988. eCollection 2022. Front Genet. 2022. PMID: 36457748 Free PMC article.
References
- Alexeev D, Alexeeva M, Baxter RL, Campopiano DJ, Webster SP, Sawyer L (1998) The crystal structure of 8-amino-7-oxononanoate synthase: a bacterial PLP-dependent, acyl-CoA-condensing enzyme. J Mol Biol 284: 401–419 - PubMed
- Andrews NC (1999) Disorders of iron metabolism. N Engl J Med 341: 1986–1995 - PubMed
- Bolt EL, Kryszak L, Zeilstra-Ryalls J, Shoolingin-Jordan PM, Warren MJ (1999) Characterization of the Rhodobacter sphaeroides 5-aminolaevulinic acid synthase isoenzymes, HemA and HemT, isolated from recombinant Escherichia coli. Eur J Biochem 265: 290–299 - PubMed
- Bottomley SS (2004) Sideroblastic anemias. In Wintrobe's Clinical Hematology, Greer J, Foerster J, Lukens JN, Rodgers GM, Paraskevas R, Glader Bertil (eds), pp 1012–1033. Philadelphia: Lippincott Williams & Wilkins
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL (1998) Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr 54 (Part 5): 905–921 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials