Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer - PubMed (original) (raw)
Comparative Study
Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer
Holbrook E Kohrt et al. PLoS Med. 2005 Sep.
Abstract
Background: While lymph node metastasis is among the strongest predictors of disease-free and overall survival for patients with breast cancer, the immunological nature of tumor-draining lymph nodes is often ignored, and may provide additional prognostic information on clinical outcome.
Methods and findings: We performed immunohistochemical analysis of 47 sentinel and 104 axillary (nonsentinel) nodes from 77 breast cancer patients with 5 y of follow-up to determine if alterations in CD4, CD8, and CD1a cell populations predict nodal metastasis or disease-free survival. Sentinel and axillary node CD4 and CD8 T cells were decreased in breast cancer patients compared to control nodes. CD1a dendritic cells were also diminished in sentinel and tumor-involved axillary nodes, but increased in tumor-free axillary nodes. Axillary node, but not sentinel node, CD4 T cell and dendritic cell populations were highly correlated with disease-free survival, independent of axillary metastasis. Immune profiling of ALN from a test set of 48 patients, applying CD4 T cell and CD1a dendritic cell population thresholds of CD4 > or = 7.0% and CD1a > or = 0.6%, determined from analysis of a learning set of 29 patients, provided significant risk stratification into favorable and unfavorable prognostic groups superior to clinicopathologic characteristics including tumor size, extent or size of nodal metastasis (CD4, p < 0.001 and CD1a, p < 0.001). Moreover, axillary node CD4 T cell and CD1a dendritic cell populations allowed more significant stratification of disease-free survival of patients with T1 (primary tumor size 2 cm or less) and T2 (5 cm or larger) tumors than all other patient characteristics. Finally, sentinel node immune profiles correlated primarily with the presence of infiltrating tumor cells, while axillary node immune profiles appeared largely independent of nodal metastases, raising the possibility that, within axillary lymph nodes, immune profile changes and nodal metastases represent independent processes.
Conclusion: These findings demonstrate that the immune profile of tumor-draining lymph nodes is of novel biologic and clinical importance for patients with early stage breast cancer.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
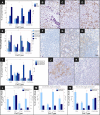
Figure 1. Lymph Node Profile of Sentinel and Axillary Lymph Nodes
Mean and standard error of CD4 and CD8 T cell, CD1a dendritic cell populations as percent of lymph node, and CD4:CD8 cell ratio are shown for (A) SLN (n = 29), ALN (n = 29), and control lymph nodes (n = 10); (E) tumor-involved ALNs (n = 9), tumor-free ALNs (n = 7) from patients with a positive ALND, tumor-free ALNs from patients with a negative ALND (n = 13), and controls (n = 10); (I) SLNs and ALNs stratified by disease recurrence during 5 y of follow-up (11 of 29 with recurrent disease); (L) tumor-involved ALNs stratified by disease recurrence (n = 9); (M) tumor-free ALNs from patients with a positive ALND stratified by disease recurrence (n = 7); and (N) tumor-free ALNs from patients with a negative ALND stratified by disease recurrence (n = 13). Representative 200× images of lymphocyte population (brown staining) and infiltrating tumor (purple staining) by IHC, including CD8 T cells in (B) SLNs, (C) ALNs, and (D) controls; (F) CD4 T cells in tumor-involved ALNs, (G) tumor-free ALNs from patients with a positive ALND, (H) tumor-free ALNs from patients with a negative ALND; and (J) CD1a dendritic cells in ALNs from patients disease-free versus (K) patients who developed recurrence.
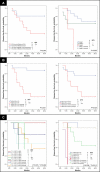
Figure 2. Disease-free Survival Analysis of Women with Breast Cancer According to Immune profile Characteristics, Learning Set ALN Series 2, and Test Set
KM curves are shown for (A) median DFS applied to the learning set ALN series 2 (n = 27) and test set (n = 48) according to size of CD4 T cell and CD1a dendritic cell populations within learning set ALN series 2 (second, randomly selected ALN per individual); (B) DFS stratified by size of CD4 T cell and CD1a dendritic cell populations within test set ALNs; and (C) DFS applied to the learning set (n = 29) and test set (n = 48) according to size of ALN CD4 T cell and ALN CD1a dendritic cell populations. Thresholds for ALN CD4 T cell and ALN CD1a dendritic cell populations were determined by ROC curves as applied to the learning set (ALN series 1). Median duration of DFS are indicated; – indicates a median DFS greater than follow-up period, 5 y. Of 29 individuals in learning set ALN series 1, 11 had recurrent disease, and of 27 individuals in learning set ALN series 2, 11 had recurrent disease. Of 48 individuals in the test set of ALNs, 22 had recurrent disease. For ALN selection from the learning set (C), a single ALN was randomly selected from series 1 or series 2 per individual. Adjusted _p_-values were determined by the permuted log-rank statistic for comparison of DFS between groups.
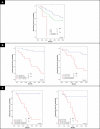
Figure 3. DFS Analysis of Women with Breast Cancer According to Tumor and Immune profile Characteristics, Learning Set ALN Series 1
KM curves are shown for (A) median DFS applied to the learning set, n = 29, according to percent of SLN occupied by infiltrating tumor (determined by IHC), and stratified by tumor stage; (B) DFS according to size of CD4 T cell and CD1a dendritic cell populations within learning set ALN series 1 (first, arbitrarily selected ALN per individual); and (C) DFS stratified both by percent of SLN infiltrated by tumor and tumor stage, and by both axillary node CD4 T cell population and by tumor stage. A comparison of survival by all subgroups and a separate comparison of stratified T2 alone are included (* in [C]). Thresholds for percent tumor infiltration within SLN, ALN CD4 T cell, and ALN CD1a dendritic cell populations were determined by ROC curves as applied to the learning set (SLN and ALN series 1). Median duration of DFS are indicated; – indicates a median DFS greater than follow-up period, 5 y. Of 29 individuals, 11 had recurrent disease. Adjusted _p-_values were determined by the permuted log-rank statistic for comparison of disease-free survival between groups. TI, tumor infiltration.
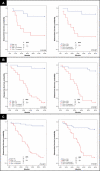
Figure 4. DFS Analysis of Women with Breast Cancer According to Tumor Stage, T1 and T2, and Immune Profile Characteristics, Learning and Test Sets
KM curves are shown for (A) median DFS applied to the learning set (n = 29) and test set (n = 48) according to tumor stage; (B) DFS stratified by size of ALN CD4 T cell and ALN CD1a dendritic cell populations among individuals with T1 tumors; and (C) DFS stratified by size of ALN CD4 T cell and ALN CD1a dendritic cell populations among individuals with T2 tumors. Thresholds for ALN CD4 T cell and ALN CD1a dendritic cell populations were determined by receiver-operating-characteristic curves as applied to the learning set (ALN series 1). Median duration of DFS are indicated; – indicates a median DFS greater than follow-up period, 5 y. Of 77 individuals, 33 had disease recurrence. Of 41 from individuals with T1 tumors, 15 had recurrent disease. Of 33 individuals with T2 tumors, 15 had disease recurrence. For ALN selection from the learning set, a single ALN was randomly selected from series 1 or series 2 per individual. Adjusted _p_-values were determined by the permuted log-rank statistic for comparison of DFS between groups.
Similar articles
- [Optimal treatment of the axilla after positive sentinel lymph node biopsy in early invasive breast cancer. Early results of the OTOASOR trial].
Sávolt A, Musonda P, Mátrai Z, Polgár C, Rényi-Vámos F, Rubovszky G, Kovács E, Sinkovics I, Udvarhelyi N, Török K, Kásler M, Péley G. Sávolt A, et al. Orv Hetil. 2013 Dec 8;154(49):1934-42. doi: 10.1556/OH.2013.29765. Orv Hetil. 2013. PMID: 24292111 Clinical Trial. Hungarian. - Histopathologic validation of the sentinel lymph node hypothesis for breast carcinoma.
Turner RR, Ollila DW, Krasne DL, Giuliano AE. Turner RR, et al. Ann Surg. 1997 Sep;226(3):271-6; discussion 276-8. doi: 10.1097/00000658-199709000-00006. Ann Surg. 1997. PMID: 9339933 Free PMC article. Clinical Trial. - Evaluation of immunohistochemistry and multiple-level sectioning in sentinel lymph nodes from patients with breast cancer.
Pargaonkar AS, Beissner RS, Snyder S, Speights VO Jr. Pargaonkar AS, et al. Arch Pathol Lab Med. 2003 Jun;127(6):701-5. doi: 10.5858/2003-127-701-EOIAMS. Arch Pathol Lab Med. 2003. PMID: 12741893 - Sentinel lymph node as a new marker for therapeutic planning in breast cancer patients.
Gipponi M, Bassetti C, Canavese G, Catturich A, Di Somma C, Vecchio C, Nicolò G, Schenone F, Tomei D, Cafiero F. Gipponi M, et al. J Surg Oncol. 2004 Mar;85(3):102-11. doi: 10.1002/jso.20022. J Surg Oncol. 2004. PMID: 14991881 Review. - Staging of women with breast cancer after introduction of sentinel node guided axillary dissection.
Tvedskov TF. Tvedskov TF. Dan Med J. 2012 Jul;59(7):B4475. Dan Med J. 2012. PMID: 22759850 Review.
Cited by
- Tumor draining lymph nodes connected to cold triple-negative breast cancers are characterized by Th2-associated microenvironment.
Guo W, Tan J, Wang L, Egelston CA, Simons DL, Ochoa A, Lim MH, Wang L, Solomon S, Waisman J, Wei CH, Hoffmann C, Song J, Schmolze D, Lee PP. Guo W, et al. Nat Commun. 2024 Oct 4;15(1):8592. doi: 10.1038/s41467-024-52577-y. Nat Commun. 2024. PMID: 39366933 Free PMC article. - Two Different Immune Profiles Are Identified in Sentinel Lymph Nodes of Early-Stage Breast Cancer.
Ribeiro JM, Mendes J, Gante I, Figueiredo-Dias M, Almeida V, Gomes A, Regateiro FJ, Regateiro FS, Caramelo F, Silva HC. Ribeiro JM, et al. Cancers (Basel). 2024 Aug 19;16(16):2881. doi: 10.3390/cancers16162881. Cancers (Basel). 2024. PMID: 39199652 Free PMC article. - Antibody-activation of connexin hemichannels in bone osteocytes with ATP release suppresses breast cancer and osteosarcoma malignancy.
Riquelme MA, Wang X, Acosta FM, Zhang J, Chavez J, Gu S, Zhao P, Xiong W, Zhang N, Li G, Srinivasan S, Ma C, Rao MK, Sun LZ, Zhang N, An Z, Jiang JX. Riquelme MA, et al. Cell Rep. 2024 Jul 23;43(7):114377. doi: 10.1016/j.celrep.2024.114377. Epub 2024 Jun 17. Cell Rep. 2024. PMID: 38889005 Free PMC article. - The Tumor Immune Microenvironment in Breast Cancer Progression.
Otterlei Fjørtoft M, Huse K, Rye IH. Otterlei Fjørtoft M, et al. Acta Oncol. 2024 May 23;63:359-367. doi: 10.2340/1651-226X.2024.33008. Acta Oncol. 2024. PMID: 38779867 Free PMC article. Review. - B Cells and IL-21-Producing Follicular Helper T Cells Cooperate to Determine the Dynamic Alterations of Premetastatic Tumor Draining Lymph Nodes of Breast Cancer.
Mao X, Tang X, Pan H, Yu M, Ji S, Qiu W, Che N, Zhang K, Huang Z, Jiang Y, Wang J, Zhong Z, Wang J, Liu M, Chen M, Zhou W, Wang S. Mao X, et al. Research (Wash D C). 2024 Mar 29;7:0346. doi: 10.34133/research.0346. eCollection 2024. Research (Wash D C). 2024. PMID: 38559676 Free PMC article.
References
- Badwe RA, Thorat MA, Parmar VV. Sentinel-node biopsy in breast cancer. N Engl J Med. 2003;349:1968–1971. - PubMed
- Page DL, Jensen RA, Simpson JF. Routinely available indicators of prognosis in breast cancer. Breast Cancer Res Treat. 1998;51:195–208. - PubMed
- Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546–553. - PubMed
- Krag D, Weaver D, Ashikaga T, Moffat F, Klimberg VS, et al. The sentinel node in breast cancer—A multicenter validation study. N Engl J Med. 1998;339:941–946. - PubMed
- Veronesi U, Paganelli G, Galimberti V, Viale G, Zurrida S, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997;349:1864–1867. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials