Formation of human myocardium in the rat heart from human embryonic stem cells - PubMed (original) (raw)
Formation of human myocardium in the rat heart from human embryonic stem cells
Michael A Laflamme et al. Am J Pathol. 2005 Sep.
Abstract
Human embryonic stem cells (hESCs) offer the opportunity to replenish cells lost in the postinfarct heart. We explored whether human myocardium could be generated in rat hearts by injecting differentiated cardiac-enriched hESC progeny into the left ventricular wall of athymic rats. Although initial grafts were predominantly epithelial, noncardiac elements were lost over time, and grafts consisted predominantly of cardiomyocytes by 4 weeks. No teratomatous elements were observed. Engrafted cardiomyocytes were glycogen-rich and expressed expected cardiac markers including beta-myosin heavy chain, myosin light chain 2v, and atrial natriuretic factor. Heat-shock treatment improved graft size approximately threefold. The cardiac implants exhibited substantial angiogenesis, both recipient and graft derived. Importantly, there was greater proliferation in human cardiomyocytes than previously seen in rodent-derived cardiomyocytes: 14.4% of graft cardiomyocytes expressed the proliferation marker Ki-67, and 2.7% incorporated the thymidine analog BrdU 4 weeks after transplantation. This proliferation was associated with a sevenfold increase in graft size over the 4-week interval. Thus, hESCs can form human myocardium in the rat heart, permitting studies of human myocardial development and physiology and supporting the feasibility of their use in myocardial repair.
Figures
Figure 1
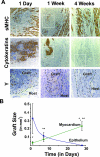
Time course study of human ESC cardiomyocyte grafts. A: The three top panels show immunostaining (brown deposit) for sarcomeric myosin heavy chain (sMHC) at 1 day, 1 week, and 4 weeks after transplantation and identify host and graft cardiomyocytes. The three middle panels show immunostaining for epithelial cells (pan-cytokeratins) on adjacent sections. Note that, over time, the tightly clustered human myocardial implants (enclosed within dotted lines) appear to have expanded, whereas epithelial elements decrease in extent and are totally absent by 4 weeks. The lineage of the engrafted human cells is confirmed in the three bottom panels, in which adjacent sections have undergone in situ hybridization with a human-specific probe against the Y-chromosome (punctate intranuclear dots), these from fields corresponding to the boxes in the above panels. Scale bar = 50 μm; counterstain is hematoxylin. Y in situ images are magnified an additional 2.5-fold. B: The plot shows the changing composition of the graft versus time (n = 4 to 10 hearts per marker per time point). Whereas the mean total epithelial (ie, pan-cytokeratin+; blue line) graft cross-sectional area declines with time and is totally absent by 4 weeks, the mean total cardiac graft area shows a sevenfold increase from the 1-day to 4-week time points. Note that the mean cardiac graft area at 4 weeks shows a statistically significant difference from that at both the 1-day (*) and 1-week (**) time points by Student’s _t_-test (P < 0.05).
Figure 2
Additional phenotyping of human ESC cardiomyocyte grafts. All images depict 4-week-old grafts. A: Clustered human ESC-derived cardiomyocyte graft cells are positive for sarcomeric actin (sarc Actin, immunostain indicated by red deposit) and are identified by their reactivity for the human-specific Y-chromosome probe by in situ hybridization (Y, punctate brown intranuclear signal, best seen in inset magnified an additional threefold). B: The peculiar appearance of the engrafted cardiac cells by routine H&E stain in a serial section, by which they show a distinctly vacuolated appearance. This pattern is likely accounted for by the results depicted C, wherein engrafted cardiomyocytes were found to be intensely PAS reactive. This PAS reaction is completely removed by preceding amylase digestion (PAS+Amylase), as depicted in D, indicating engrafted cardiomyocytes are heavily glycogen-laden. E: The cardiomyocyte graft cells stained positive for the ventricular-specific marker myosin light chain 2v (MLC2V). As depicted by sarcomeric myosin heavy chain (sMHC) immunostain in F, the engrafted cardiomyocytes assume sarcomeric banding and alignment with host fibers (inset magnified an additional twofold). G and H: Adjacent sections immunostained with antibodies against connexin-43 (Cx43) and pan-cadherins (Cadh), respectively. The inset in G shows in situ hybridization with the human-specific pan-centromeric probe (on adjacent section), thus identifying a clump of human ESC-derived cardiomyocytes residing within the indicated box. This clump of cells showed the expected strong reactivity with β-myosin heavy chain (not shown). Note the total absence of connexin-43 reactivity, despite the expected intercalated disk staining pattern on the surrounding host myocardium. In contrast, this same field does show definite diffuse membranous staining of the human cardiac implant with anti-pan-cadherins. Scale bars = 50 μm.
Figure 3
Human ESC-derived cardiac grafts show both recipient- and graft-derived angiogenesis. A: A typical cardiac implant 4 weeks after transplantation; the graft myocardium is readily distinguished from that of the host by its strong immunoreactivity for β-myosin heavy chain (β-MHC) and by in situ hybridization on an adjacent section with a human-specific pan-centromeric probe (HC, within inset). The ingrowth of both rat-derived and human-derived (ie, graft) vessels into this cardiac implant is demonstrated in B through D, which contain adjacent tissue sections stained with species-specific endothelial markers. B and D: Human endothelial cells, as detected by staining with U. europaeus agglutinin I and a human-specific anti-CD31 antibody, respectively. C: Rat endothelial cells, as defined by the rat-specific pan-endothelial antibody RECA-1, which marks the microvasculature of both the cardiac implant and the surrounding host myocardium. Scale bar = 100 μm; insets magnified an additional 1.5-fold. Sections are counterstained with hematoxylin, except for the inset of A, which is counterstained with Fast Green.
Figure 4
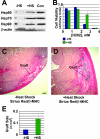
Heat-shock pretreatment is cytoprotective and yields large grafts. A: The expression of heat-shock proteins in H7 hESC-derived, Percoll-fractionated cardiomyocytes, as determined by Western blot analysis with antibodies against Hsp60, Hsp70, and Hsp90. Note the induction of all three isoforms in heat-shocked cells (lane +HS) over untreated controls (lane −HS). Heat-shocked HeLa cell lysate (lane Con) was included as positive control. Expression of β-actin was measured to control for protein loading. B: The protective effect of heat shock on hESC-derived cardiomyocytes is examined by quantifying (by MTS assay) the survival of control (blue) and heat-shocked (green) Percoll-fractionated cells in the face of varying concentrations of hydrogen peroxide (n = 2 preparations per condition). Note the enhanced cell survival in response to heat shock at the intermediate peroxide concentration. C and D: Human ESC-derived cardiac implants and associated fibrosis in hearts receiving control and heat-shocked cells, respectively. Fields contain representative islands of β-myosin heavy chain+ human cardiomyocytes (blue/black deposit), each taken from the heart with the median-sized graft from its corresponding experimental group. As was typical, cardiac grafts were present on a background of Sirius Red-positive scar tissue (intense red staining, with staining specificity subsequently confirmed under polarized light). E: Heat-shock treatment (+HS) yielded a statistically significant, ≅3-fold difference in cardiac graft volume over non-heat-shocked cells (−HS). Scale bar = 200 μm.
Figure 5
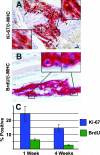
Human ESC-derived cardiac grafts are highly proliferative. A: A 4-week-old cardiac implant is double-immunostained for β-myosin heavy chain (red deposit), the predominant myosin isoform in the human cardiomyocytes, and a human-specific antibody against the proliferative marker Ki-67 (brown intranuclear staining). Note that the host myocardium is β-myosin heavy chain negative. Representative Ki-67+ cardiomyocyte nuclei are indicated by the insets (magnified an additional threefold) and arrows. B: Another 4-week-old implant is double-immunostained for β-myosin heavy chain (red deposit) and anti-BrdU (brown intranuclear staining). Two clear-cut BrdU+ and β-myosin heavy chain+ human cardiomyocytes are indicated by the arrow and right inset. In addition, occasional mitotic figures were noted within the human cardiomyocytes, as illustrated by the left inset. Magnification, ×400 (insets magnified an additional threefold). C: 1 week after transplantation, 28.3 ± 5.4% of β-myosin heavy chain-positive graft cells were Ki-67+ and 6.4 ± 0.8% were BrdU+. As long as 4 weeks after transplantation, a still robust 15.8 ± 3.4% of graft cardiomyocytes were Ki-67+ and 2.7 ± 0.3% were BrdU+. Scale bar = 100 μm.
Similar articles
- Survival and maturation of human embryonic stem cell-derived cardiomyocytes in rat hearts.
Dai W, Field LJ, Rubart M, Reuter S, Hale SL, Zweigerdt R, Graichen RE, Kay GL, Jyrala AJ, Colman A, Davidson BP, Pera M, Kloner RA. Dai W, et al. J Mol Cell Cardiol. 2007 Oct;43(4):504-16. doi: 10.1016/j.yjmcc.2007.07.001. Epub 2007 Jul 14. J Mol Cell Cardiol. 2007. PMID: 17707399 Free PMC article. - Cardiomyocytes derived from embryonic stem cells resemble cardiomyocytes of the embryonic heart tube.
Fijnvandraat AC, van Ginneken AC, de Boer PA, Ruijter JM, Christoffels VM, Moorman AF, Lekanne Deprez RH. Fijnvandraat AC, et al. Cardiovasc Res. 2003 May 1;58(2):399-409. doi: 10.1016/s0008-6363(03)00282-7. Cardiovasc Res. 2003. PMID: 12757874 - Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts.
Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Caspi O, et al. J Am Coll Cardiol. 2007 Nov 6;50(19):1884-93. doi: 10.1016/j.jacc.2007.07.054. Epub 2007 Oct 23. J Am Coll Cardiol. 2007. PMID: 17980256 - Cardiac application of embryonic stem cells.
Xiao YF. Xiao YF. Sheng Li Xue Bao. 2003 Oct 25;55(5):493-504. Sheng Li Xue Bao. 2003. PMID: 14566394 Review. - Stem cells in cardiac repair.
Henning RJ. Henning RJ. Future Cardiol. 2011 Jan;7(1):99-117. doi: 10.2217/fca.10.109. Future Cardiol. 2011. PMID: 21174514 Review.
Cited by
- Simulated Microgravity and 3D Culture Enhance Induction, Viability, Proliferation and Differentiation of Cardiac Progenitors from Human Pluripotent Stem Cells.
Jha R, Wu Q, Singh M, Preininger MK, Han P, Ding G, Cho HC, Jo H, Maher KO, Wagner MB, Xu C. Jha R, et al. Sci Rep. 2016 Aug 5;6:30956. doi: 10.1038/srep30956. Sci Rep. 2016. PMID: 27492371 Free PMC article. - Human embryonic stem cells: Distinct molecular personalities and applications in regenerative medicine.
Dupont G, Yilmaz E, Loukas M, Macchi V, De Caro R, Tubbs RS. Dupont G, et al. Clin Anat. 2019 Apr;32(3):354-360. doi: 10.1002/ca.23318. Epub 2019 Jan 7. Clin Anat. 2019. PMID: 30521112 Free PMC article. Review. - The current status of engineering myocardial tissue.
Sui R, Liao X, Zhou X, Tan Q. Sui R, et al. Stem Cell Rev Rep. 2011 Mar;7(1):172-80. doi: 10.1007/s12015-010-9131-8. Stem Cell Rev Rep. 2011. PMID: 20198517 Review. - Nanowired human cardiac organoid transplantation enables highly efficient and effective recovery of infarcted hearts.
Tan Y, Coyle RC, Barrs RW, Silver SE, Li M, Richards DJ, Lin Y, Jiang Y, Wang H, Menick DR, Deleon-Pennell K, Tian B, Mei Y. Tan Y, et al. Sci Adv. 2023 Aug 4;9(31):eadf2898. doi: 10.1126/sciadv.adf2898. Epub 2023 Aug 4. Sci Adv. 2023. PMID: 37540743 Free PMC article. - Stem cells for heart cell therapies.
Jing D, Parikh A, Canty JM Jr, Tzanakakis ES. Jing D, et al. Tissue Eng Part B Rev. 2008 Dec;14(4):393-406. doi: 10.1089/ten.teb.2008.0262. Tissue Eng Part B Rev. 2008. PMID: 18821841 Free PMC article.
References
- Hassink RJ, Brutel de, la Riviere A, Mummery CL, Doevendans PA. Transplantation of cells for cardiac repair. J Am Coll Cardiol. 2003;41:711–717. - PubMed
- Hassink RJ, Dowell JD, Brutel de, la Riviere A, Doevendans PA, Field LJ. Stem cell therapy for ischemic heart disease. Trends Mol Med. 2003;9:436–441. - PubMed
- Murry CE, Whitney ML, Laflamme MA, Reinecke H, Field LJ. Cellular therapies for myocardial infarct repair. Cold Spring Harb Symp Quant Biol. 2002;67:519–526. - PubMed
- Jain M, DerSimonian H, Brenner DA, Ngoy S, Teller P, Edge AS, Zawadzka A, Wetzel K, Sawyer DB, Colucci WS, Apstein CS, Liao R. Cell therapy attenuates deleterious ventricular remodeling and improves cardiac performance after myocardial infarction. Circulation. 2001;103:1920–1927. - PubMed
Publication types
MeSH terms
Grants and funding
- HL07828-06/HL/NHLBI NIH HHS/United States
- T32 HL007828/HL/NHLBI NIH HHS/United States
- R01 HL064387/HL/NHLBI NIH HHS/United States
- P01 HL003174/HL/NHLBI NIH HHS/United States
- R24 HL064387/HL/NHLBI NIH HHS/United States
- HL03174/HL/NHLBI NIH HHS/United States
- HL64387/HL/NHLBI NIH HHS/United States
- R01 HL061553/HL/NHLBI NIH HHS/United States
- HL61553/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical