Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo - PubMed (original) (raw)
Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo
Michele A Grimbaldeston et al. Am J Pathol. 2005 Sep.
Abstract
Mice carrying certain mutations in the white spotting (W) locus (ie, c-kit) exhibit reduced c-kit tyrosine kinase-dependent signaling that results in mast cell deficiency and other phenotypic abnormalities. The c-kit mutations in Kit(W/W-v) mice impair melanogenesis and result in anemia, sterility, and markedly reduced levels of tissue mast cells. In contrast, Kit(W-sh/W-sh) mice, bearing the W-sash (W(sh)) inversion mutation, have mast cell deficiency but lack anemia and sterility. We report that adult Kit(W-sh/W-sh) mice had a profound deficiency in mast cells in all tissues examined but normal levels of major classes of other differentiated hematopoietic and lymphoid cells. Unlike Kit(W/W-v) mice, Kit(W-sh/W-sh) mice had normal numbers of TCR gammadelta intraepithelial lymphocytes in the intestines and did not exhibit a high incidence of idiopathic dermatitis, ulcers, or squamous papillomas of the stomach, but like Kit(W/W-v) mice, they lacked interstitial cells of Cajal in the gut and exhibited bile reflux into the stomach. Systemic or local reconstitution of mast cell populations was achieved in nonirradiated adult Kit(W-sh/W-sh) mice by intravenous, intraperitoneal, or intradermal injection of wild-type bone marrow-derived cultured mast cells but not by transplantation of wild-type bone marrow cells. Thus, Kit(W-sh/W-sh) mice represent a useful model for mast cell research, especially for analyzing mast cell function in vivo.
Figures
Figure 1
Levels of hematopoietic cells in adult c-kit mutant C57BL/6-Kit W-sh/W-sh mice and the congenic wild-type littermates. For analysis of each lineage, three to four mice per group were investigated, with very similar results obtained for all of the mice within each group. The plots presented in this figure are the results obtained from one mouse chosen as representative of the data from each group. A: B-cell populations in spleen and bone marrow. B: CD4+ and CD8+ T cells in thymus and spleen. C: Kit W-sh/W-sh mice exhibit normal numbers of small intestinal TCRγδ and TCRαβ IELs, whereas Kit W/W-v mice exhibit depletion of TCRγδ IELs and a corresponding increase in TCRαβ IELs. D: Myeloid cell populations (granulocytes and macrophages) in bone marrow, spleen, and peritoneal cavity. E: CD11c+ dendritic cell and CD49b+ natural killer cell populations in spleen (NKT cell populations were not specifically analyzed). F: c-Kitlo FcεRIα+ basophils in bone marrow and spleen.
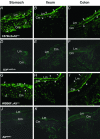
Figure 2
Morphological identification of c-Kit expressing ICC in the stomach (A, D, G, J), ileum (B, E, H, K), and colon (C, F, I, L) in wild-type C57BL/6-Kit+/+ (A–C) and WBB6F1-Kit+/+ mice (G–I), but ICC were not detected in mutant C57BL/6-Kit W-sh/W-sh (D–F) or WBB6F1-Kit W/Wv mice (J–L). A and G: Cryostat cross-sections show detection of ICC within the longitudinal muscle (Lm) and in the circular muscle (Cm) layers of the gastric fundus, orientated parallel to longitudinal muscle cells (arrowheads) and observed in cross-section within the circular muscle bundles (arrows). B and H: Cross-sections through the ileum reveal ICC between the Lm and Cm at the level of the myenteric plexus (arrowheads) and at the level of the deep muscular plexus (arrows). C and I: ICC in the proximal colon were identified at the level of the myenteric plexus (arrowheads) within the Cm (small arrow) and along the submucosal surface of the circular muscle layer (arrows). Original magnifications, ×400.
Figure 3
Mast cell numbers (per mm horizontal length of skin or per mm2) in dorsal (back) skin (A); ear pinna (B); peritoneal cavity (percent mast cells in lavage) (C); mesenteric window (D); jejunum, ileum, and colon (E); stomach (F); lung parenchyma (G); and spleen (H) of C57BL/6-Kit+/+ (black diamond), Kit W-sh/W-sh (white diamond) and Kit+/+ (gray triangle), or GFP+ (gray diamond), or Ly5.2+ (gray circle) BMCMC-engrafted Kit W-sh/W-sh mice. Samples of tissues were obtained 6 to 8 weeks after intraperitoneal or intradermal or 12 weeks after intravenous adoptive transfer of BMCMCs. The mean for each group is indicated on the graphs. *, **, and ***: P < 0.05, 0.01, and 0.001,respectively, versus corresponding values for mast cell-deficient Kit W-sh/W-sh mice. Supplementary Table 2 at http://ajp.amjpathol.org shows the mean ± SD data for mast cell numbers in all tissues/sites examined in each group, as well as the number of mice per group and additional statistical analysis of the results.
Figure 4
Histological sections showing the dermis and dermal fat of back skin (A–C), mesenteric windows (D–F), and the submucosa and muscularis propria of the glandular stomach (E-I) in C57BL/6-Kit+/+ (A, D, G), Kit W-sh/W-sh (B, E, H), and mast cell knockin Kit W-sh/W-sh mice that had been injected with congenic GFP+ BMCMCs intradermally (C), intraperitoneally (F), or intravenously (I). Mast cells were not detected in the majority of tissue sections from Kit W-sh/W-sh mice, but a few mast cells were sometimes detected in back skin. Arrows: mast cells. Original magnifications: ×200; ×1000 (insets).
Similar articles
- Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient Kit(W-sh)/Kit(W-sh) sash mice.
Wolters PJ, Mallen-St Clair J, Lewis CC, Villalta SA, Baluk P, Erle DJ, Caughey GH. Wolters PJ, et al. Clin Exp Allergy. 2005 Jan;35(1):82-8. doi: 10.1111/j.1365-2222.2005.02136.x. Clin Exp Allergy. 2005. PMID: 15649271 Free PMC article. - Genetic inversion in mast cell-deficient (Wsh) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy.
Nigrovic PA, Gray DH, Jones T, Hallgren J, Kuo FC, Chaletzky B, Gurish M, Mathis D, Benoist C, Lee DM. Nigrovic PA, et al. Am J Pathol. 2008 Dec;173(6):1693-701. doi: 10.2353/ajpath.2008.080407. Epub 2008 Nov 6. Am J Pathol. 2008. PMID: 18988802 Free PMC article. - Mast cell-deficient Kit(W-sh) "Sash" mutant mice display aberrant myelopoiesis leading to the accumulation of splenocytes that act as myeloid-derived suppressor cells.
Michel A, Schüler A, Friedrich P, Döner F, Bopp T, Radsak M, Hoffmann M, Relle M, Distler U, Kuharev J, Tenzer S, Feyerabend TB, Rodewald HR, Schild H, Schmitt E, Becker M, Stassen M. Michel A, et al. J Immunol. 2013 Jun 1;190(11):5534-44. doi: 10.4049/jimmunol.1203355. Epub 2013 May 1. J Immunol. 2013. PMID: 23636054 - The kit-ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogenesis.
Besmer P, Manova K, Duttlinger R, Huang EJ, Packer A, Gyssler C, Bachvarova RF. Besmer P, et al. Dev Suppl. 1993:125-37. Dev Suppl. 1993. PMID: 7519481 Review. - Analyzing mast cell development and function using mice carrying mutations at W/c-kit or Sl/MGF (SCF) loci.
Galli SJ, Tsai M, Gordon JR, Geissler EN, Wershil BK. Galli SJ, et al. Ann N Y Acad Sci. 1992;664:69-88. doi: 10.1111/j.1749-6632.1992.tb39750.x. Ann N Y Acad Sci. 1992. PMID: 1280935 Review.
Cited by
- SNAP23 is essential for platelet and mast cell development and required in connective tissue mast cells for anaphylaxis.
Cardenas RA, Gonzalez R, Sanchez E, Ramos MA, Cardenas EI, Rodarte AI, Alcazar-Felix RJ, Isaza A, Burns AR, Heidelberger R, Adachi R. Cardenas RA, et al. J Biol Chem. 2021 Jan-Jun;296:100268. doi: 10.1016/j.jbc.2021.100268. Epub 2021 Jan 8. J Biol Chem. 2021. PMID: 33837726 Free PMC article. - Susceptibility to vaccinia virus infection and spread in mice is determined by age at infection, allergen sensitization and mast cell status.
Domenico J, Lucas JJ, Fujita M, Gelfand EW. Domenico J, et al. Int Arch Allergy Immunol. 2012;158(2):196-205. doi: 10.1159/000330647. Epub 2012 Jan 26. Int Arch Allergy Immunol. 2012. PMID: 22286752 Free PMC article. - Analyzing the Functions of Mast Cells In Vivo Using 'Mast Cell Knock-in' Mice.
Gaudenzio N, Sibilano R, Starkl P, Tsai M, Galli SJ, Reber LL. Gaudenzio N, et al. J Vis Exp. 2015 May 27;(99):e52753. doi: 10.3791/52753. J Vis Exp. 2015. PMID: 26068439 Free PMC article. - Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells.
Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, Mitchell AJ, Tay SS, Jain R, Forbes-Blom E, Chen X, Tong PL, Bolton HA, Artis D, Paul WE, Fazekas de St Groth B, Grimbaldeston MA, Le Gros G, Weninger W. Roediger B, et al. Nat Immunol. 2013 Jun;14(6):564-73. doi: 10.1038/ni.2584. Epub 2013 Apr 21. Nat Immunol. 2013. PMID: 23603794 Free PMC article. - Apoptotic cells suppress mast cell inflammatory responses via the CD300a immunoreceptor.
Nakahashi-Oda C, Tahara-Hanaoka S, Shoji M, Okoshi Y, Nakano-Yokomizo T, Ohkohchi N, Yasui T, Kikutani H, Honda S, Shibuya K, Nagata S, Shibuya A. Nakahashi-Oda C, et al. J Exp Med. 2012 Jul 30;209(8):1493-503. doi: 10.1084/jem.20120096. Epub 2012 Jul 23. J Exp Med. 2012. PMID: 22826299 Free PMC article.
References
- Kitamura Y, Go S, Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978;52:447–452. - PubMed
- Kitamura Y. Heterogeneity of mast cells and phenotypic change between subpopulations. Annu Rev Immunol. 1989;7:59–76. - PubMed
- Russell ES. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. - PubMed
- Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CMM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI023990/AI/NIAID NIH HHS/United States
- AI23990/AI/NIAID NIH HHS/United States
- R01 CA072074/CA/NCI NIH HHS/United States
- P50 HL067674/HL/NHLBI NIH HHS/United States
- CA72074/CA/NCI NIH HHS/United States
- R37 AI023990/AI/NIAID NIH HHS/United States
- P01 HL067674/HL/NHLBI NIH HHS/United States
- HL67674/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases