Abnormalities of GATA-1 in megakaryocytes from patients with idiopathic myelofibrosis - PubMed (original) (raw)
Abnormalities of GATA-1 in megakaryocytes from patients with idiopathic myelofibrosis
Alessandro M Vannucchi et al. Am J Pathol. 2005 Sep.
Abstract
The abnormal megakaryocytopoiesis associated with idiopathic myelofibrosis (IM) plays a role in its pathogenesis. Because mice with defective expression of transcription factor GATA-1 (GATA-1(low) mutants) eventually develop myelofibrosis, we investigated the occurrence of GATA-1 abnormalities in IM patients. CD 34(+) cells were purified from 12 IM patients and 8 controls; erythroblasts and megakaryocytes were then obtained from unilineage cultures of CD 34(+) cells. Purified CD 61(+), GPA(+), and CD 34(+) cells from IM patients contained levels of GATA-1, GATA-2, and FOG-1 mRNA, as well as of GATA-2 protein, that were similar to controls. In contrast, CD 61(+) cells from IM patients contained significantly reduced GATA-1 protein. Furthermore, 45% of megakaryocytes in biopsies from IM patients did not stain with anti-GATA-1 antibody, as compared to controls (2%), essential thrombocythemia (4%), or polycythemia vera (11%) patients. Abnormalities in immunoreactivity for FOG-1 were not found, and no mutations in GATA-1 coding sequences were found. The presence of GATA-1(neg) megakaryocytes in bone marrow biopsies was independent of the Val 617 Phe JAK 2 mutation, making it unlikely that a downstream functional relationship exists. We conclude that megakaryocytes from IM patients have reduced GATA-1 content, possibly contributing to disease pathogenesis as in the GATA-1(low) mice and also representing a novel IM-associated marker.
Figures
Figure 1
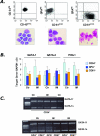
Analysis of GATA-1 mRNA in CD34+ cells purified from the peripheral blood of IM patients or the BM of controls, and in their erythroid (GPA+) or megakaryocyte (CD61+) progeny obtained in vitro. A: Purity of the isolated cell fractions (CD34+, GPA+, CD61+, from the left) was re-evaluated by fluorescence-activated cell sorting analysis (top), whereas morphological appearance was assessed on May-Grümwald/Giemsa-stained cytosmears (bottom). B: Quantification of GATA-1, GATA-2, and FOG-1 mRNA levels by real-time RT-PCR analysis. A total of 8 controls (for all different cell populations), and 12, 11, and 10 IM patients, respectively, for CD34+, GPA+, and CD61+ cells were analyzed. Results are expressed as mean (±SD; each sample assayed in triplicate) ΔCt for each target gene after normalization for the amplification level of the housekeeping GAPDH gene; lower ΔCt values indicate higher target gene expression levels. C: RT-PCR amplification of the full-length (GATA-1f) and the shorter (GATA-1s) GATA-1 transcript starting from mRNA of CD34+, GPA+, and CD61+ cells. The results are representative of those obtained with cells purified from a total of four controls and seven IM patients. Amplicons were analyzed during the exponential phase of PCR (cycles = 31). The lane on the left contains molecular weight markers (marker IX).
Figure 2
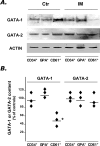
Western blot analysis of GATA-1 and GATA-2 protein in purified CD34+, GPA+, and CD61+ cells obtained from IM patients and controls. A: Due to the low amount of protein recovered, pools of three IM patients or four controls were prepared by mixing equal numbers of cells purified from each patient. A representative blot from one normal and one IM pool is shown in A, while the percentage changes of GATA-1 and GATA-2 protein content in the three IM pools (as compared to the two control pools), after normalization for the actin content, is shown in B. Horizontal lines indicate the mean values. *P = 0.02. Films in A were exposed for different times (5 minutes for GATA-1 and GATA-2, 45 seconds for actin).
Figure 3
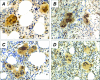
Immunohistochemistry analysis of GATA-1 in BM biopsies from IM patients (B, G) or controls (A, F). In A, all morphologically recognizable megakaryocytes (filled arrows) are strongly stained in the nucleus, with some cytoplasmic staining. On the other hand, the heterogeneity typically observed in IM samples, with a combination of megakaryocytes normally stained (filled arrows) or showing no to very faint nucleus/cytoplasmic staining (open arrows) (GATA-1neg megakaryocytes) can be appreciated in B. The staining pattern in PV or ET patients is shown in C and D, respectively. The percentage of GATA-1neg megakaryocytes in controls (n = 10) and in patients with either IM (n = 17), ET, or PV (n = 10 for both) is shown in E. Early erythroid cells in a typical erythroid island of a normal patient (F) are heavily stained, whereas more mature erythroblasts show weaker staining; the same pattern can be appreciated in the section from an IM patient (G), in which GATA-1pos erythroblasts appear surrounded by GATA-1neg megakaryocytes (open arrows). Original magnifications, ×200.
Figure 4
Immunohistochemistry analysis of FOG-1 in BM biopsies from controls (A) or patients with IM (B), PV (C), or ET (D). Megakaryocytes and rare erythroid cells appear strongly and similarly stained with FOG-1 antibody. Original magnifications, ×400.
Figure 5
JAK2 mutation analysis in patients with IM (A), PV (B), or ET (C). The presence of the Val617Phe JAK2 mutation was analyzed by an allele-specific PCR; the 203-bp product is specifically amplified from the mutant allele, while the 364-bp product serves as an internal control for both mutant and normal allele. The percentage of GATA-1neg megakaryocytes according to the presence or not of JAK2 mutation is reported in D.
Similar articles
- The role of beta-catenin in chronic myeloproliferative disorders.
Jauregui MP, Sanchez SR, Ewton AA, Rice L, Perkins SL, Dunphy CH, Chang CC. Jauregui MP, et al. Hum Pathol. 2008 Oct;39(10):1454-8. doi: 10.1016/j.humpath.2008.02.007. Epub 2008 Jul 11. Hum Pathol. 2008. PMID: 18619646 - A pathobiologic pathway linking thrombopoietin, GATA-1, and TGF-beta1 in the development of myelofibrosis.
Vannucchi AM, Bianchi L, Paoletti F, Pancrazzi A, Torre E, Nishikawa M, Zingariello M, Di Baldassarre A, Rana RA, Lorenzini R, Alfani E, Migliaccio G, Migliaccio AR. Vannucchi AM, et al. Blood. 2005 May 1;105(9):3493-501. doi: 10.1182/blood-2004-04-1320. Epub 2005 Jan 21. Blood. 2005. PMID: 15665119 - Impaired GATA-1 expression and myelofibrosis in an animal model.
Vannucchi AM, Bianchi L, Paoletti F, Di Giacomo V, Migliaccio G, Migliaccio AR. Vannucchi AM, et al. Pathol Biol (Paris). 2004 Jun;52(5):275-9. doi: 10.1016/j.patbio.2004.02.008. Pathol Biol (Paris). 2004. PMID: 15217713 Review. - Pathogenesis of myelofibrosis with myeloid metaplasia: lessons from mouse models of the disease.
Vannucchi AM, Migliaccio AR, Paoletti F, Chagraoui H, Wendling F. Vannucchi AM, et al. Semin Oncol. 2005 Aug;32(4):365-72. doi: 10.1053/j.seminoncol.2005.04.008. Semin Oncol. 2005. PMID: 16202682 Review. - Proposal for revised diagnostic criteria of essential thrombocythemia and polycythemia vera by the Thrombocythemia Vera Study Group.
Michiels JJ, Juvonen E. Michiels JJ, et al. Semin Thromb Hemost. 1997;23(4):339-47. doi: 10.1055/s-2007-996107. Semin Thromb Hemost. 1997. PMID: 9263350
Cited by
- The Role of Megakaryocytes in Myelofibrosis.
Melo-Cardenas J, Migliaccio AR, Crispino JD. Melo-Cardenas J, et al. Hematol Oncol Clin North Am. 2021 Apr;35(2):191-203. doi: 10.1016/j.hoc.2020.11.004. Epub 2021 Jan 11. Hematol Oncol Clin North Am. 2021. PMID: 33641863 Free PMC article. Review. - Lysyl oxidase is associated with increased thrombosis and platelet reactivity.
Matsuura S, Mi R, Koupenova M, Eliades A, Patterson S, Toselli P, Thon J, Italiano JE Jr, Trackman PC, Papadantonakis N, Ravid K. Matsuura S, et al. Blood. 2016 Mar 17;127(11):1493-501. doi: 10.1182/blood-2015-02-629667. Epub 2016 Jan 11. Blood. 2016. PMID: 26755713 Free PMC article. - GATA1 Is a Sensitive and Specific Nuclear Marker for Erythroid and Megakaryocytic Lineages.
Lee WY, Weinberg OK, Pinkus GS. Lee WY, et al. Am J Clin Pathol. 2017 Apr 1;147(4):420-426. doi: 10.1093/ajcp/aqx018. Am J Clin Pathol. 2017. PMID: 28340113 Free PMC article. - mTOR inhibitors alone and in combination with JAK2 inhibitors effectively inhibit cells of myeloproliferative neoplasms.
Bogani C, Bartalucci N, Martinelli S, Tozzi L, Guglielmelli P, Bosi A, Vannucchi AM; Associazione Italiana per la Ricerca sul Cancro AGIMM Gruppo Italiano Malattie Mieloproliferative. Bogani C, et al. PLoS One. 2013;8(1):e54826. doi: 10.1371/journal.pone.0054826. Epub 2013 Jan 31. PLoS One. 2013. PMID: 23382981 Free PMC article. - Loss of the Gata1 gene IE exon leads to variant transcript expression and the production of a GATA1 protein lacking the N-terminal domain.
Kobayashi E, Shimizu R, Kikuchi Y, Takahashi S, Yamamoto M. Kobayashi E, et al. J Biol Chem. 2010 Jan 1;285(1):773-83. doi: 10.1074/jbc.M109.030726. Epub 2009 Oct 23. J Biol Chem. 2010. PMID: 19854837 Free PMC article.
References
- Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–2302. - PubMed
- Tefferi A. The forgotten myeloproliferative disorder: myeloid metaplasia. Oncologist. 2003;8:225–231. - PubMed
- O’Brien S, Tefferi A, Valent P. Chronic myelogenous leukemia and myeloproliferative disease. Hematology (Am Soc Hematol Educ Program) 2004:146–162. - PubMed
- Hoffman R. Agnogenic myeloid metaplasia. Hoffman R, Benz EJ, Shattil SJ, Furie B, Cohen H, Silberstein LE, McGlave P, editors. New York: Churchill Livingstone,; Hematology. Basic Principles and Practice. 2000:pp 1172–1188.
- Xu M, Bruno E, Chao J, Ni H, Lindgren V, Nunez R, Mahmud N, Finazzi G, Fruchtman SM, Popat U, Liu E, Prchal JT, Rondelli D, Barosi G, Hoffman R. The constitutive mobilization of bone marrow repopulating cells into the peripheral blood in idiopathic myelofibrosis. Blood. 2005;105:4508–4515. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources