Plx1 is the 3F3/2 kinase responsible for targeting spindle checkpoint proteins to kinetochores - PubMed (original) (raw)
Plx1 is the 3F3/2 kinase responsible for targeting spindle checkpoint proteins to kinetochores
Oi Kwan Wong et al. J Cell Biol. 2005.
Abstract
Dynamic attachment of microtubules to kinetochores during mitosis generates pulling force, or tension, required for the high fidelity of chromosome separation. A lack of tension activates the spindle checkpoint and delays the anaphase onset. A key step in the tension-response pathway involves the phosphorylation of the 3F3/2 epitope by an unknown kinase on untensed kinetochores. Using a rephosphorylation assay in Xenopus laevis extracts, we identified the kinetochore-associated Polo-like kinase Plx1 as the kinase both necessary and sufficient for this phosphorylation. Indeed, Plx1 is the physiological 3F3/2 kinase involved in checkpoint response, as immunodepletion of Plx1 from checkpoint extracts abolished the 3F3/2 signal and blocked association of xMad2, xBubR1, xNdc80, and xNuf2 with kinetochores. Interestingly, the kinetochore localization of Plx1 is under the control of the checkpoint protein xMps1, as immunodepletion of xMps1 prevents binding of Plx1 to kinetochores. Thus, Plx1 couples the tension signal to cellular responses through phosphorylating the 3F3/2 epitope and targeting structural and checkpoint proteins to kinetochores.
Figures
Figure 1.
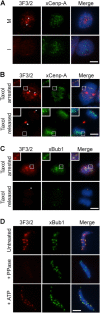
The 3F3/2 epitope in X. laevis is a phosphospecific kinetochore antigen. (A) Asynchronous XTC cells in mitosis (M) and in interphase (I) were stained with the 3F3/2 antibody and with an anti–xCenp-A antibody. The majority of the xCenp-A dots in M cells contained the 3F3/2 signals, although a few lacked the 3F3/2 staining, likely because of the dephosphorylation that occurred during sample processing. (B and C) XTC cells arrested with taxol or released from taxol arrest were stained for the 3F3/2 antigen and for xCenp-A or xBub1. Insets show a magnified image of the boxed areas. (D) Nuclei purified from checkpoint extracts (top) were dephosphorylated with λ-phosphatase (middle) and then rephosphorylated with ATP (bottom). (A–D) Red, 3F3/2; green, xCenp-A or xBub1. Arrowheads and arrow point to the 3F3/2 signals on centrosomes and at the midbody, respectively. Bars, 5 μm.
Figure 2.
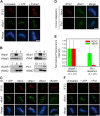
Plx1 is both necessary and sufficient to phosphorylate the 3F3/2 epitope at kinetochores. (A) Nuclei purified from checkpoint extracts carrying the 3F3/2 epitope (left) were dephosphorylated, treated with NEM, and rephosphorylated with either ATP (middle) or ATP plus CSF extract (right). Nuclei were then stained for the 3F3/2 epitope and xBub1. (B) Immunodepletion of kinetochore-associated kinases from CSF extracts. CSF extracts were depleted of xMps1 (lane 1), xBub1 (lane 4), xBubR1 (lane 8), and Plx1 (lane 11). 1 μl of depleted extracts and mock-depleted extracts (lanes 2, 5, 9, and 12) and 0.05 μl of input extracts (lanes 3, 6, 7, and 10) were analyzed by Western blotting to determine the depletion efficiency. xMad1 and xMad2 were shown here to demonstrate the specificity of the immunodepletion. (C and D) Rephosphorylation of 3F3/2 by extracts depleted of kinetochore-associated kinases. Nuclei were prepared as in A and rephosphorylated with either ATP (first column in C) or ATP plus depleted extracts prepared in B. ID, immunodepletion. (E) Mean kinetochore fluorescence intensity of xBub1 (green) and 3F3/2 (red) signals from samples rephosphorylated with xBubR1- or Plx1-depleted extracts. The fluorescence intensity was normalized to the corresponding values derived from mock-depleted extracts. Error bars represent SD. (F) Nuclei from checkpoint extracts were dephosphorylated and rephosphorylated with ATP or with ATP plus recombinant His6-Plx1. (A, C, D, and F) Red, 3F3/2; green, xBub1. Bars, 5 μm.
Figure 3.
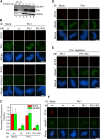
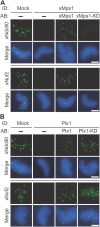
Plx1 is the physiological 3F3/2 kinase. (A) Extracts were mock depleted (lane 6) or depleted of Plx1 (lanes 3–5). Plx1 (lane 4) or Plx1-KD (lane 5) was translated in CSF extracts that had been depleted of endogenous Plx1 and then added to the Plx1-depleted extracts. Lanes 1 and 2 show input extracts. The volumes of extracts loaded were as indicated. Different amounts of input extracts were loaded to quantify the degree of depletion and add-back. (B and D–F) 3F3/2, Plx1, xCenp-A, xMad2, and xBubR1 staining of nuclei purified from checkpoint extracts that had undergone immunodepletion (ID) and add-back (AB) of the indicated proteins. Red, 3F3/2; green, Plx1, xCenp-A, xMad2, and xBubR1. (C) Mean kinetochore fluorescence intensity (from 15 randomly selected kinetochores) of xBubR1 (green) and 3F3/2 (red) signals from samples that were depleted of Plx1 and then added back with the indicated proteins. The fluorescence intensity was normalized to the corresponding values derived from mock-depleted extracts. Error bars represent SD. Bars, 5 μm.
Figure 4.
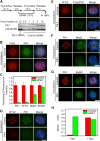
Plk1 is required for the generation of the 3F3/2 epitope in HeLa cells. (A, top) Schematic of double-thymidine synchronization and siRNA transfection of HeLa cells. (bottom) Western blot analysis of Plk1 and control knockdown cell lysates to determine the knockdown efficiency. p38 MAPK was used as a loading control. The volumes of HeLa cell lysates loaded were as indicated. Different amounts of cell lysates were loaded from the control knockdown sample to quantify the degree of Plk1 knockdown. (B and D–G) Plk1 or control knockdown cells were fixed at 11 h after release from the second thymindine arrest. Prometaphase cells were stained with the following antibodies: (B) Crest serum (red) and anti-Plk1 (green); (D) 3F3/2 antibody (red) and anti-Plk1 (green); (E) 3F3/2 antibody (red), Crest serum (green), and anti-Plk1 (green); (F) Plk1 (red) and Mad2 (green); (G) Plk1 (red) and BubR1 (green). In D, F, and G, arrowheads point to 3F3/2 and Plk1 signals at spindle poles. Bars, 5 μm. (C) Mean kinetochore fluorescence intensity (from randomly selected kinetochores of multiple prometaphase cells) of Plk1, 3F3/2, Mad2, and BubR1 signals from Plk1 (green) or control knockdown (red) cells. The fluorescence intensity was normalized to the corresponding values derived from control knockdown cells. Error bars represent SD. (H) HeLa cells were synchronized by double-thymidine arrest/release and transfected with siRNAs as described in A, except that transfected cells were released from the second thymidine arrest in either the presence (+ Noc) or absence (− Noc) of 100 ng/ml nocodazole. At 14 h after release from the second thymidine arrest, cells were fixed, and the mitotic index was counted (n > 150 cells for each sample).
Figure 5.
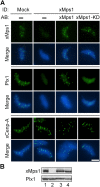
xMps1 controls the kinetochore localization of Plx1. (A) CSF extracts were either mock depleted or depleted of xMps1 and then added back with the indicated proteins. Nuclei were purified from depleted checkpoint extracts and stained for xMps1, Plx1, and xCenp-A. ID, immunodepletion; AB, add-back. Bar, 5 μm. (B) Depletion efficiency was determined by Western blot analysis of equal volumes of mock- (lane 1) or xMps1-depleted (lane 2) extracts as well as xMps1-depleted extracts with the add-back of xMps1 (lane 3) or xMps1-KD (lane 4).
Figure 6.
xMps1 and Plx1 are required for the assembly of the outer kinetochore structure. (A) Nuclei were purified from mock-, xMps1-, or xMps1-depleted extracts with the add-back of the indicated proteins, as described in Fig. 5. Purified nuclei were stained for xNdc80 or xNuf2. (B) Nuclei were purified from mock-, Plx1-, or Plx1-depleted extracts with the add-back of the indicated proteins, as described in Fig. 3. Purified nuclei were stained for xNdc80 and xNuf2. ID, immunodepletion; AB, add-back. Bars, 5 μm.
Similar articles
- Loading of the 3F3/2 antigen onto kinetochores is dependent on the ordered assembly of the spindle checkpoint proteins.
Wong OK, Fang G. Wong OK, et al. Mol Biol Cell. 2006 Oct;17(10):4390-9. doi: 10.1091/mbc.e06-04-0346. Epub 2006 Aug 2. Mol Biol Cell. 2006. PMID: 16885416 Free PMC article. - Polo-like kinase 1 creates the tension-sensing 3F3/2 phosphoepitope and modulates the association of spindle-checkpoint proteins at kinetochores.
Ahonen LJ, Kallio MJ, Daum JR, Bolton M, Manke IA, Yaffe MB, Stukenberg PT, Gorbsky GJ. Ahonen LJ, et al. Curr Biol. 2005 Jun 21;15(12):1078-89. doi: 10.1016/j.cub.2005.05.026. Curr Biol. 2005. PMID: 15964272 - Cdk1 phosphorylation of BubR1 controls spindle checkpoint arrest and Plk1-mediated formation of the 3F3/2 epitope.
Wong OK, Fang G. Wong OK, et al. J Cell Biol. 2007 Nov 19;179(4):611-7. doi: 10.1083/jcb.200708044. Epub 2007 Nov 12. J Cell Biol. 2007. PMID: 17998400 Free PMC article. - The spindle checkpoint in Xenopus laevis.
Chen RH. Chen RH. Front Biosci. 2008 Jan 1;13:2231-7. doi: 10.2741/2837. Front Biosci. 2008. PMID: 17981705 Review. - Cell cycle: checkpoint proteins and kinetochores.
Straight AF. Straight AF. Curr Biol. 1997 Oct 1;7(10):R613-6. doi: 10.1016/s0960-9822(06)00315-0. Curr Biol. 1997. PMID: 9368739 Review.
Cited by
- Requirements for protein phosphorylation and the kinase activity of polo-like kinase 1 (Plk1) for the kinetochore function of mitotic arrest deficiency protein 1 (Mad1).
Chi YH, Haller K, Ward MD, Semmes OJ, Li Y, Jeang KT. Chi YH, et al. J Biol Chem. 2008 Dec 19;283(51):35834-44. doi: 10.1074/jbc.M804967200. Epub 2008 Oct 15. J Biol Chem. 2008. PMID: 18922800 Free PMC article. - The spindle checkpoint and chromosome segregation in meiosis.
Gorbsky GJ. Gorbsky GJ. FEBS J. 2015 Jul;282(13):2471-87. doi: 10.1111/febs.13166. Epub 2015 Jan 12. FEBS J. 2015. PMID: 25470754 Free PMC article. Review. - Polo-like kinases: conservation and divergence in their functions and regulation.
Archambault V, Glover DM. Archambault V, et al. Nat Rev Mol Cell Biol. 2009 Apr;10(4):265-75. doi: 10.1038/nrm2653. Nat Rev Mol Cell Biol. 2009. PMID: 19305416 Review. - Welcome to a new kind of tension: translating kinetochore mechanics into a wait-anaphase signal.
Maresca TJ, Salmon ED. Maresca TJ, et al. J Cell Sci. 2010 Mar 15;123(Pt 6):825-35. doi: 10.1242/jcs.064790. J Cell Sci. 2010. PMID: 20200228 Free PMC article. Review. - Mutations in Drosophila Greatwall/Scant reveal its roles in mitosis and meiosis and interdependence with Polo kinase.
Archambault V, Zhao X, White-Cooper H, Carpenter AT, Glover DM. Archambault V, et al. PLoS Genet. 2007 Nov;3(11):e200. doi: 10.1371/journal.pgen.0030200. PLoS Genet. 2007. PMID: 17997611 Free PMC article.
References
- Abrieu, A., L. Magnaghi-Jaulin, J.A. Kahana, M. Peter, A. Castro, S. Vigneron, T. Lorca, D.W. Cleveland, and J.C. Labbâe. 2001. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell. 106:83–93. - PubMed
- Ahonen, L.J., M.J. Kallio, J.R. Daum, M. Bolton, I.A. Manke, M.B. Yaffe, P.T. Stukenberg, and G.J. Gorbsky. 2005. Polo-like kinase 1 creates the tension-sensing 3F3/2 phosphoepitope and modulates the association of spindle-checkpoint proteins at kinetochores. Curr. Biol. 15:1078–1089. - PubMed
- Barr, F.A., H.H. Sillje, and E.A. Nigg. 2004. Polo-like kinases and the orchestration of cell division. Nat. Rev. Mol. Cell Biol. 5:429–440. - PubMed
- Campbell, M.S., J.R. Daum, M.S. Gersch, R.B. Nicklas, and G.J. Gorbsky. 2000. Kinetochore “memory” of spindle checkpoint signaling in lysed mitotic cells. Cell Motil. Cytoskeleton. 46:146–156. - PubMed